Phase I and II randomized clinical trial of an oral therapeutic vaccine targeting human papillomavirus for treatment of cervical intraepithelial neoplasia 2 and 3
- PMID: 38001029
- PMCID: PMC10748578
- DOI: 10.1093/jncics/pkad101
Phase I and II randomized clinical trial of an oral therapeutic vaccine targeting human papillomavirus for treatment of cervical intraepithelial neoplasia 2 and 3
Abstract
Background: Although many human papillomavirus (HPV)-targeted therapeutic vaccines have been examined for efficacy in clinical trials, none have been translated into clinical use. These previous agents were mostly administered by intramuscular or subcutaneous injection to induce systemic immunity. We investigated the safety and therapeutic efficacy of an HPV-16 E7-expressing lacticaseibacillus-based oral vaccine.
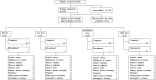
Methods: In a double-blind, placebo-controlled, randomized trial, a total of 165 patients with HPV-16-positive high-grade cervical intraepithelial neoplasia 2 and 3 were assigned to orally administered placebo or low, intermediate, or high doses of IGMKK16E7 (lacticaseibacillus paracasei expressing cell surface, full-length HPV-16 E7). In the 4 groups, IGMKK16E7 or placebo was administered orally at weeks 1, 2, 4, and 8 postenrollment. The primary outcomes included histopathological regression and IGMKK16E7 safety.
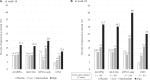
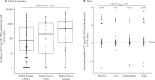
Results: In per-protocol analyses, histopathological regression to normal (complete response) occurred in 13 (31.7%) of 41 high-dose recipients and in 5 (12.5%) of 40 placebo recipients (rate difference = 19.2, 95% confidence interval [CI] = 0.5 to 37.8). In patients positive for HPV-16 only, the clinical response rate was 40.0% (12 of 30) in high-dose recipients and 11.5% (3 of 26) in recipients of placebo (rate difference = 28.5, 95% CI = 4.3 to 50.0). There was no difference in adverse events that occurred in the high-dose and placebo groups (P = .83). The number of HPV-16 E7-specific interferon-γ producing cells within peripheral blood increased with level of response (stable disease, partial, and complete responses; P = .004). The regression to normal (complete response) rates among recipients with high levels of immune response were increased in a dose-dependent manner.
Conclusion: This trial demonstrates safety of IGMKK16E7 and its efficacy against HPV-16-positive cervical intraepithelial neoplasia 2 and 3. IGMKK16E7 is the first oral immunotherapeutic vaccine to show antineoplastic effects.
Trial registration: jRCT2031190034.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Dr Kawana reports receiving lecture fees and travel support from MSD and grant support from the Japan Agency for Medical and Development (AMED) and GLOVACC Co Ltd; Dr Uemura, lecture fees from Chugai and Eli Lilly; Dr Igimi, patent indemnification from National Institute of Health Sciences and grant support from GLOVACC Co Ltd; Dr Schust, unrelated grant support from the National Institutes of Health. No other potential conflict of interest relevant to this article were reported.
Figures

Similar articles
-
Safety and efficacy of mucosal immunotherapy using human papillomavirus (HPV) type 16 E7-expressing Lactobacillus-based vaccine for the treatment of high-grade squamous intraepithelial lesion (HSIL): the study protocol of a randomized placebo-controlled clinical trial (MILACLE study).Jpn J Clin Oncol. 2019 Sep 1;49(9):877-880. doi: 10.1093/jjco/hyz095. Jpn J Clin Oncol. 2019. PMID: 31613356 Clinical Trial.
-
Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial.Lancet. 2015 Nov 21;386(10008):2078-2088. doi: 10.1016/S0140-6736(15)00239-1. Epub 2015 Sep 17. Lancet. 2015. PMID: 26386540 Free PMC article. Clinical Trial.
-
Optimization of human papillomavirus (HPV) type 16 E7-expressing lactobacillus-based vaccine for induction of mucosal E7-specific IFNγ-producing cells.Vaccine. 2018 Jun 7;36(24):3423-3426. doi: 10.1016/j.vaccine.2018.05.009. Epub 2018 May 5. Vaccine. 2018. PMID: 29735324
-
Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials.Expert Rev Vaccines. 2018 Dec;17(12):1085-1091. doi: 10.1080/14760584.2018.1548282. Epub 2018 Nov 29. Expert Rev Vaccines. 2018. PMID: 30495978 Review.
-
Cell-mediated immune response: a clinical review of the therapeutic potential of human papillomavirus vaccination.Acta Obstet Gynecol Scand. 2014 Dec;93(12):1209-18. doi: 10.1111/aogs.12480. Epub 2014 Sep 20. Acta Obstet Gynecol Scand. 2014. PMID: 25146484 Review.
Cited by
-
Serum levels of stearic and dihomo-γ-linolenic acids can be used to diagnose cervical cancer and cervical intraepithelial neoplasia.Sci Rep. 2024 Sep 6;14(1):20833. doi: 10.1038/s41598-024-71606-w. Sci Rep. 2024. PMID: 39242718 Free PMC article.
-
Low CD86 expression is a predictive biomarker for clinical response to the therapeutic human papillomavirus vaccine IGMKK16E7: results of a post hoc analysis.JNCI Cancer Spectr. 2024 Nov 1;8(6):pkae091. doi: 10.1093/jncics/pkae091. JNCI Cancer Spectr. 2024. PMID: 39302712 Free PMC article. Clinical Trial.
-
Therapeutic Vaccines for HPV-Associated Cervical Malignancies: A Systematic Review.Vaccines (Basel). 2024 Apr 17;12(4):428. doi: 10.3390/vaccines12040428. Vaccines (Basel). 2024. PMID: 38675811 Free PMC article. Review.
-
Current status and future directions for the development of human papillomavirus vaccines.Front Immunol. 2024 Jun 25;15:1362770. doi: 10.3389/fimmu.2024.1362770. eCollection 2024. Front Immunol. 2024. PMID: 38983849 Free PMC article. Review.
-
The Past, Present, and Future of Cervical Cancer Vaccines.Vaccines (Basel). 2025 Feb 17;13(2):201. doi: 10.3390/vaccines13020201. Vaccines (Basel). 2025. PMID: 40006746 Free PMC article. Review.
References
-
- Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453-e463. - PubMed
-
- Bruni L, Saura-Lázaro A, Montoliu A,. et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med. 2021;144:106399. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
