Impact of Intravenous Fat Emulsion Choice on Candida Biofilm, Hyphal Growth, and Catheter-Related Bloodstream Infections in Pediatric Patients
- PMID: 38001054
- PMCID: PMC10873182
- DOI: 10.1093/infdis/jiad527
Impact of Intravenous Fat Emulsion Choice on Candida Biofilm, Hyphal Growth, and Catheter-Related Bloodstream Infections in Pediatric Patients
Abstract
Background: Use of mixed-oil (MO) intravenous fat emulsion (IFE) was shown to inhibit Candida albicans biofilm formation and overall rate of catheter-related bloodstream infections (CR-BSIs) compared with soybean-oil (SO) IFE). We aimed to delineate this inhibitory mechanism and impact of IFE choice on distribution of fungal CR-BSIs.
Methods: Transcriptional profiling was conducted on C. albicans grown in SO-IFE, MO-IFE, or SO-IFE with capric acid. Overexpression strains of shared down-regulated genes were constructed using a tetracycline-off system to assess hypha and biofilm formation in IFEs. A 5-year retrospective multicenter cohort study was performed to assess differences in CR-BSIs caused by Candida species based on the IFE formulation received in pediatric patients.
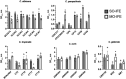
Results: Genes significantly down-regulated in MO-IFE and SO-IFE with capric acid included CDC11, HGC1, and UME6. Overexpression of HGC1 or UME6 enabled filamentation in capric acid and MO-IFE. Interestingly, only overexpression of UME6 was sufficient to rescue biofilm growth in MO-IFE. MO-IFE administration was associated with a higher proportion of non-albicans Candida versus C. albicans CR-BSIs (42% vs 33%; odds ratio, 1.22 [95% confidence interval, .46-3.26]).
Conclusions: MO-IFE affects C. albicans biofilm formation and hyphal growth via a UME6-dependent mechanism. A numerical but not statistically significant difference in distribution of Candida spp. among CR-BSIs was observed.
Keywords: Candida; biofilm; catheter-related infections; intravenous fat emulsions; parenteral nutrition.
Plain language summary
Delivery of carbohydrates, amino acids, and lipids via intravenous catheters is necessary for some patients to supply daily caloric needs. These nutrient-dense parenteral solutions can promote microbial biofilm growth on the catheter surface, which may seed subsequent catheter-related bloodstream infection (CR-BSI). In fact, receipt of parenteral nutrition is an established risk factor for CR-BSI caused by the polymorphic fungal pathogen Candida albicans. New intravenous fat emulsions (IFEs) have gained market share and IFEs containing capric acid (mixed-oil [MO] IFE) compared with those without (soybean-oil [SO] IFE) impair the C. albicans yeast-to-hypha switch—a trait strongly associated with pathogenicity and biofilm formation. In this study, we found that MO-IFE and capric acid reduced expression of a transcriptional regulator involved in hyphal extension (UME6) and down-regulated genes involved in cell partitioning (HGC1). Overexpression of these genes enabled hyphal growth in MO-IFE. Secondly, we sought to determine whether the type of IFE administered was associated with the clinical incidence of CR-BSIs caused by C. albicans or other common non-albicans Candida species. There was a nonsignificant numerical reduction in C. albicans infections in patients administered MO-IFE compared with SO-IFE. Collectively, this work shows that IFEs differentially affect Candida biology with potential infectious consequences for the patient.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest . All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



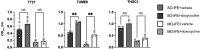
References
-
- Netto R, Mondini M, Pezzella C, et al. . Parenteral nutrition is one of the most significant risk factors for nosocomial infections in a pediatric cardiac intensive care unit. J Parenter Enteral Nutr 2017; 41:612–8. - PubMed
-
- Pappas PG, Kauffman CA, Andes DR, et al. . Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:409–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

