Cytolytic circumsporozoite-specific memory CD4+ T cell clones are expanded during Plasmodium falciparum infection
- PMID: 38001069
- PMCID: PMC10673885
- DOI: 10.1038/s41467-023-43376-y
Cytolytic circumsporozoite-specific memory CD4+ T cell clones are expanded during Plasmodium falciparum infection
Abstract
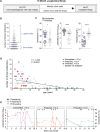
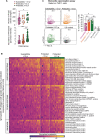
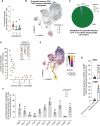
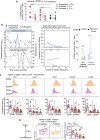
Clinical immunity against Plasmodium falciparum infection develops in residents of malaria endemic regions, manifesting in reduced clinical symptoms during infection and in protection against severe disease but the mechanisms are not fully understood. Here, we compare the cellular and humoral immune response of clinically immune (0-1 episode over 18 months) and susceptible (at least 3 episodes) during a mild episode of Pf malaria infection in a malaria endemic region of Malawi, by analysing peripheral blood samples using high dimensional mass cytometry (CyTOF), spectral flow cytometry and single-cell transcriptomic analyses. In the clinically immune, we find increased proportions of circulating follicular helper T cells and classical monocytes, while the humoral immune response shows characteristic age-related differences in the protected. Presence of memory CD4+ T cell clones with a strong cytolytic ZEB2+ T helper 1 effector signature, sharing identical T cell receptor clonotypes and recognizing the Pf-derived circumsporozoite protein (CSP) antigen are found in the blood of the Pf-infected participants gaining protection. Moreover, in clinically protected participants, ZEB2+ memory CD4+ T cells express lower level of inhibitory and chemotactic receptors. We thus propose that clonally expanded ZEB2+ CSP-specific cytolytic memory CD4+ Th1 cells may contribute to clinical immunity against the sporozoite and liver-stage Pf malaria.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- World Health Organization. World Malaria Report 2021 (WHO, 2021).
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

