Lipolysis supports bone formation by providing osteoblasts with endogenous fatty acid substrates to maintain bioenergetic status
- PMID: 38001111
- PMCID: PMC10673934
- DOI: 10.1038/s41413-023-00297-2
Lipolysis supports bone formation by providing osteoblasts with endogenous fatty acid substrates to maintain bioenergetic status
Abstract
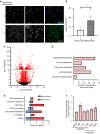
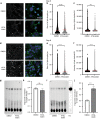
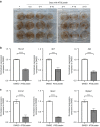
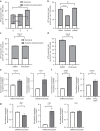
Bone formation is a highly energy-demanding process that can be impacted by metabolic disorders. Glucose has been considered the principal substrate for osteoblasts, although fatty acids are also important for osteoblast function. Here, we report that osteoblasts can derive energy from endogenous fatty acids stored in lipid droplets via lipolysis and that this process is critical for bone formation. As such, we demonstrate that osteoblasts accumulate lipid droplets that are highly dynamic and provide the molecular mechanism by which they serve as a fuel source for energy generation during osteoblast maturation. Inhibiting cytoplasmic lipolysis leads to both an increase in lipid droplet size in osteoblasts and an impairment in osteoblast function. The fatty acids released by lipolysis from these lipid droplets become critical for cellular energy production as cellular energetics shifts towards oxidative phosphorylation during nutrient-depleted conditions. In vivo, conditional deletion of the ATGL-encoding gene Pnpla2 in osteoblast progenitor cells reduces cortical and trabecular bone parameters and alters skeletal lipid metabolism. Collectively, our data demonstrate that osteoblasts store fatty acids in the form of lipid droplets, which are released via lipolysis to support cellular bioenergetic status when nutrients are limited. Perturbations in this process result in impairment of bone formation, specifically reducing ATP production and overall osteoblast function.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
Intracellular lipid droplets support osteoblast function.Adipocyte. 2017 Jul 3;6(3):250-258. doi: 10.1080/21623945.2017.1356505. Epub 2017 Jul 20. Adipocyte. 2017. PMID: 28792783 Free PMC article.
-
Bioenergetic Metabolism In Osteoblast Differentiation.Curr Osteoporos Rep. 2022 Feb;20(1):53-64. doi: 10.1007/s11914-022-00721-2. Epub 2022 Feb 3. Curr Osteoporos Rep. 2022. PMID: 35112289 Free PMC article. Review.
-
Subchondral bone marrow adipose tissue lipolysis regulates bone formation in hand osteoarthritis.Osteoarthritis Cartilage. 2025 Mar;33(3):322-329. doi: 10.1016/j.joca.2024.12.005. Epub 2024 Dec 24. Osteoarthritis Cartilage. 2025. PMID: 39725154
-
Energy Metabolism of the Osteoblast: Implications for Osteoporosis.Endocr Rev. 2017 Jun 1;38(3):255-266. doi: 10.1210/er.2017-00064. Endocr Rev. 2017. PMID: 28472361 Free PMC article. Review.
-
Loss of perilipin 2 in cultured myotubes enhances lipolysis and redirects the metabolic energy balance from glucose oxidation towards fatty acid oxidation.J Lipid Res. 2017 Nov;58(11):2147-2161. doi: 10.1194/jlr.M079764. Epub 2017 Aug 19. J Lipid Res. 2017. PMID: 28822960 Free PMC article.
Cited by
-
Effect of Hydrothermal Coatings of Magnesium AZ31 Alloy on Osteogenic Differentiation of hMSCs: From Gene to Protein Analysis.Materials (Basel). 2025 Mar 12;18(6):1254. doi: 10.3390/ma18061254. Materials (Basel). 2025. PMID: 40141537 Free PMC article.
-
Identification and Validation of Novel Lipids Linked to Bone Mineral Density Change and Fracture Risk.Calcif Tissue Int. 2025 Jun 25;116(1):89. doi: 10.1007/s00223-025-01399-1. Calcif Tissue Int. 2025. PMID: 40560432 Free PMC article.
-
Autophagy: regulating the seesaw of bone-fat balance.Front Cell Dev Biol. 2025 Feb 24;13:1465092. doi: 10.3389/fcell.2025.1465092. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40066259 Free PMC article. Review.
-
In C57Bl6 Mice, Obesity and Subsequent Weight Loss Negatively Affected the Skeleton and Shifted the Cortical Bone Metabolome.bioRxiv [Preprint]. 2025 May 13:2025.05.09.653133. doi: 10.1101/2025.05.09.653133. bioRxiv. 2025. PMID: 40654836 Free PMC article. Preprint.
-
Enhanced fatty acid oxidation in osteoprogenitor cells provides protection from high-fat diet induced bone dysfunction.J Bone Miner Res. 2025 Feb 2;40(2):283-298. doi: 10.1093/jbmr/zjae195. J Bone Miner Res. 2025. PMID: 39657629 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

