A self-stabilized and water-responsive deliverable coenzyme-based polymer binary elastomer adhesive patch for treating oral ulcer
- PMID: 38001112
- PMCID: PMC10673908
- DOI: 10.1038/s41467-023-43571-x
A self-stabilized and water-responsive deliverable coenzyme-based polymer binary elastomer adhesive patch for treating oral ulcer
Abstract
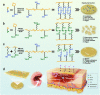
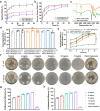
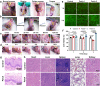
Oral ulcer can be treated with diverse biomaterials loading drugs or cytokines. However, most patients do not benefit from these materials because of poor adhesion, short-time retention in oral cavity and low drug therapeutic efficacy. Here we report a self-stabilized and water-responsive deliverable coenzyme salt polymer poly(sodium α-lipoate) (PolyLA-Na)/coenzyme polymer poly(α-lipoic acid) (PolyLA) binary synergistic elastomer adhesive patch, where hydrogen bonding cross-links between PolyLA and PolyLA-Na prevents PolyLA depolymerization and slow down the dissociation of PolyLA-Na, thus allowing water-responsive sustainable delivery of bioactive LA-based small molecules and durable adhesion to oral mucosal wound due to the adhesive action of PolyLA. In the model of mice and mini-pig oral ulcer, the adhesive patch accelerates the healing of the ulcer by regulating the damaged tissue inflammatory environment, maintaining the stability of oral microbiota, and promoting faster re-epithelialization and angiogenesis. This binary synergistic patch provided a therapeutic strategy to treat oral ulcer.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Xing JQ, et al. Barnacle-inspired robust and aesthetic Janus patch with instinctive wet adhesive for oral ulcer treatment. Chem. Eng. J. 2022;444:136580. doi: 10.1016/j.cej.2022.136580. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

