Intensive antibiotic treatment of sows with parenteral crystalline ceftiofur and tulathromycin alters the composition of the nasal microbiota of their offspring
- PMID: 38001497
- PMCID: PMC10675909
- DOI: 10.1186/s13567-023-01237-y
Intensive antibiotic treatment of sows with parenteral crystalline ceftiofur and tulathromycin alters the composition of the nasal microbiota of their offspring
Abstract
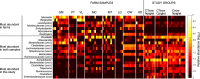
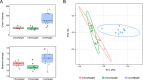
The nasal microbiota plays an important role in animal health and the use of antibiotics is a major factor that influences its composition. Here, we studied the consequences of an intensive antibiotic treatment, applied to sows and/or their offspring, on the piglets' nasal microbiota. Four pregnant sows were treated with crystalline ceftiofur and tulathromycin (CTsows) while two other sows received only crystalline ceftiofur (Csows). Sow treatments were performed at D-4 (four days pre-farrowing), D3, D10 and D17 for ceftiofur and D-3, D4 and D11 for tulathromycin. Half of the piglets born to CTsows were treated at D1 with ceftiofur. Nasal swabs were taken from piglets at 22-24 days of age and bacterial load and nasal microbiota composition were defined by 16 s rRNA gene qPCR and amplicon sequencing. Antibiotic treatment of sows reduced their nasal bacterial load, as well as in their offspring, indicating a reduced bacterial transmission from the dams. In addition, nasal microbiota composition of the piglets exhibited signs of dysbiosis, showing unusual taxa. The addition of tulathromycin to the ceftiofur treatment seemed to enhance the deleterious effect on the microbiota diversity by diminishing some bacteria commonly found in the piglets' nasal cavity, such as Glaesserella, Streptococcus, Prevotella, Staphylococcus and several members of the Ruminococcaceae and Lachnospiraceae families. On the other hand, the additional treatment of piglets with ceftiofur resulted in no further effect beyond the treatment of the sows. Altogether, these results suggest that intensive antibiotic treatments of sows, especially the double antibiotic treatment, disrupt the nasal microbiota of their offspring and highlight the importance of sow-to-piglet microbiota transmission.
Keywords: Nasal microbiota; antibiotics; bacterial transmission; piglets; sows.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Ceftiofur treatment of sows results in long-term alterations in the nasal microbiota of the offspring that can be ameliorated by inoculation of nasal colonizers.Anim Microbiome. 2023 Oct 20;5(1):53. doi: 10.1186/s42523-023-00275-3. Anim Microbiome. 2023. PMID: 37864263 Free PMC article.
-
Effect of live yeast supplementation in sow diet during gestation and lactation on sow and piglet fecal microbiota, health, and performance.J Anim Sci. 2022 Aug 1;100(8):skac209. doi: 10.1093/jas/skac209. J Anim Sci. 2022. PMID: 35675760 Free PMC article.
-
Sow Contact Is a Major Driver in the Development of the Nasal Microbiota of Piglets.Pathogens. 2021 Jun 3;10(6):697. doi: 10.3390/pathogens10060697. Pathogens. 2021. PMID: 34205187 Free PMC article.
-
Potential effect of two Bacillus probiotic strains on performance and fecal microbiota of breeding sows and their piglets.J Anim Sci. 2022 Jun 1;100(6):skac163. doi: 10.1093/jas/skac163. J Anim Sci. 2022. PMID: 35512239 Free PMC article.
-
Fermented Bamboo Fiber Improves Productive Performance by Regulating Gut Microbiota and Inhibiting Chronic Inflammation of Sows and Piglets during Late Gestation and Lactation.Microbiol Spectr. 2023 Jun 15;11(3):e0408422. doi: 10.1128/spectrum.04084-22. Epub 2023 Apr 12. Microbiol Spectr. 2023. PMID: 37042787 Free PMC article.
Cited by
-
Pig nasal and rectal microbiotas are involved in the antibody response to Glaesserella parasuis.Sci Rep. 2025 Jan 17;15(1):2347. doi: 10.1038/s41598-025-85867-6. Sci Rep. 2025. PMID: 39824862 Free PMC article.
-
Nasal microbial diversity is associated with survival in piglets infected by a highly virulent PRRSV-1 strain.Anim Microbiome. 2025 Jan 17;7(1):9. doi: 10.1186/s42523-024-00371-y. Anim Microbiome. 2025. PMID: 39825378 Free PMC article.
-
Nasal Colonizers from Sows in the Federal District of Brazil Showed a Diverse Phenotypic Resistance Profile.Microorganisms. 2025 Jun 11;13(6):1354. doi: 10.3390/microorganisms13061354. Microorganisms. 2025. PMID: 40572242 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

