Homogeneous liquid-liquid microextraction coupled with HPLC/DAD for determination of nirmatrelvir and ritonavir as COVID-19 combination therapy in human plasma
- PMID: 38001530
- PMCID: PMC10675862
- DOI: 10.1186/s13065-023-01080-4
Homogeneous liquid-liquid microextraction coupled with HPLC/DAD for determination of nirmatrelvir and ritonavir as COVID-19 combination therapy in human plasma
Abstract
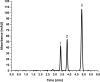
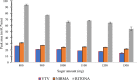
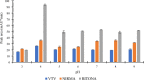
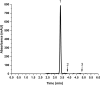
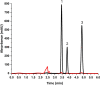
The study reports the development of a high-performance liquid chromatography/diode array detection method to measure the levels of nirmatrelvir and ritonavir in human plasma. These two antiviral medications are used for the treatment of COVID-19 and are marketed as Paxlovid®. The method employed sugaring-out induced homogeneous liquid-liquid microextraction to improve sensitivity. Optimization of the method was performed using the one variable at a time approach by adjusting several factors such as type of sugar, extractant, amount of sugar, volume of extractant, and pH of the aqueous sample to achieve the highest efficiency. The developed method was validated according to the Food and Drug Administration guidelines and demonstrated good linearity, accuracy, and precision. The range of linearity was from 1000 to 20,000 ng/mL for nirmatrelvir and 200 to 20,000 ng/mL for ritonavir with correlation coefficient values of 0.998 and 0.996, respectively. Selectivity studies revealed that no others peaks appeared in the retention times of the studied drugs. The stability of nirmatrelvir and ritonavir were also investigated through short term and three cycles of freeze-thaw, and both drugs were found stable. This analytical method could be useful for monitoring drug concentrations in patients undergoing treatment with these medications for COVID-19. In this work, for the first time, SULLME was used for the sensitive determination of nirmatrelvir and ritonavir in biological fluids. The developed method was able to determine both drugs in therapeutic levels with no need to sophisticated techniques like LC-MS. In addition to that, SULLME is considered a simple and green sample preparation in comparison with conventional sample preparation methods.
Keywords: COVID-19; HPLC; Nirmatrelvir; Ritonavir; SARS-CoV-2, Sugaring-out.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Simultaneous quantification of nirmatrelvir and ritonavir by LC-MS/MS in patients treated for COVID-19.J Chromatogr B Analyt Technol Biomed Life Sci. 2022 Dec 1;1212:123510. doi: 10.1016/j.jchromb.2022.123510. Epub 2022 Oct 17. J Chromatogr B Analyt Technol Biomed Life Sci. 2022. PMID: 36274268 Free PMC article.
-
Simultaneous quantification of nirmatrelvir/ritonavir in human serum by LC-HRMS.J Pharm Biomed Anal. 2024 Jan 5;237:115796. doi: 10.1016/j.jpba.2023.115796. Epub 2023 Oct 13. J Pharm Biomed Anal. 2024. PMID: 37839266
-
Simultaneous determination of nirmatrelvir and ritonavir in human plasma using LC-MS/MS and its pharmacokinetic application in healthy Chinese volunteers.Biomed Chromatogr. 2022 Nov;36(11):e5456. doi: 10.1002/bmc.5456. Epub 2022 Aug 5. Biomed Chromatogr. 2022. PMID: 35881032
-
Preclinical discovery and development of nirmatrelvir/ritonavir combinational therapy for the treatment of COVID-19 and the lessons learned from SARS-COV-2 variants.Expert Opin Drug Discov. 2023 Jul-Dec;18(12):1301-1311. doi: 10.1080/17460441.2023.2248879. Epub 2023 Aug 23. Expert Opin Drug Discov. 2023. PMID: 37614103 Review.
-
Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs.Clin Infect Dis. 2023 Jan 6;76(1):165-171. doi: 10.1093/cid/ciac180. Clin Infect Dis. 2023. PMID: 35245942 Free PMC article. Review.
Cited by
-
Sustainable analysis of COVID-19 Co-packaged paxlovid: exploring advanced sampling techniques and multivariate processing tools.BMC Chem. 2025 Jul 10;19(1):206. doi: 10.1186/s13065-025-01567-2. BMC Chem. 2025. PMID: 40640855 Free PMC article.
-
Development and validation of a RP-HPLC method for simultaneous determination of five COVID-19 antiviral drugs in pharmaceutical formulations.Sci Rep. 2025 Jul 15;15(1):25470. doi: 10.1038/s41598-025-09904-0. Sci Rep. 2025. PMID: 40664729 Free PMC article.
-
Stability indicating RP-HPLC technique for simultaneous estimation of nirmatrelvir and ritonavir in their new copackaged dosage form for COVID-19 treatment.Sci Rep. 2025 Jan 17;15(1):2281. doi: 10.1038/s41598-025-85776-8. Sci Rep. 2025. PMID: 39824874 Free PMC article.
References
-
- WHO . World health statistics 2022 (monitoring health of the SDGs) Geneva: WHO; 2022.
-
- Amoutzias GD, Nikolaidis M, Tryfonopoulou E, Chlichlia K, Markoulatos P, Oliver SG. The remarkable evolutionary plasticity of coronaviruses by mutation and recombination: insights for the COVID-19 pandemic and the future evolutionary paths of SARS-CoV-2. Viruses. 2022;14:78. doi: 10.3390/v14010078. - DOI - PMC - PubMed
-
- U.S. Food and Drug Administration. Fact sheet for healthcare providers emergency use authorization (EUA) for Paxlovid. US Food Drug Adm 2021:1–29.
LinkOut - more resources
Full Text Sources
Miscellaneous
