PSMC5 regulates microglial polarization and activation in LPS-induced cognitive deficits and motor impairments by interacting with TLR4
- PMID: 38001534
- PMCID: PMC10668523
- DOI: 10.1186/s12974-023-02904-9
PSMC5 regulates microglial polarization and activation in LPS-induced cognitive deficits and motor impairments by interacting with TLR4
Abstract
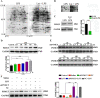
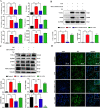
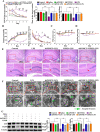
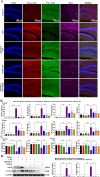
Luteolin is a flavonoid found in high concentrations in celery and green pepper, and acts as a neuroprotectant. PSMC5 (proteasome 26S subunit, ATPase 5) protein levels were reduced after luteolin stimulation in activated microglia. We aimed to determine whether regulating PSMC5 expression could inhibit neuroinflammation, and investigate the underlying mechanisms.BV2 microglia were transfected with siRNA PSMC5 before the addition of LPS (lipopolysaccharide, 1.0 µg/ml) for 24 h in serum free DMEM. A mouse model of LPS-induced cognitive and motor impairment was established to evaluate the neuroprotective effects of shRNA PSMC5. Intracerebroventricular administration of shRNA PSMC5 was commenced 7 days prior to i.p. injection of LPS (750 μg/kg). Treatments and behavioral experiments were performed once daily for 7 consecutive days. Behavioral tests and pathological/biochemical assays were performed to evaluate LPS-induced hippocampal damage. Molecular dynamics simulation was used to confirm the interaction between PSMC5 and TLR4 (Toll-like receptor 4) in LPS-stimulated BV2 microglia. SiRNA PSMC5 inhibited BV2 microglial activation, and suppressed the release of inflammatory factors (IL-1β, COX-2, PGE2, TNF-α, and iNOS) upon after LPS stimulation in BV2 microglia. LPS increased IκB-α and p65 phosphorylation, which was attenuated by siRNA PSMC5. Behavioral tests and pathological/biochemical assays showed that shRNA PSMC5 attenuated LPS-induced cognitive and motor impairments, and restored synaptic ultrastructure and protein levels in mice. ShRNA PSMC5 reduced pro-inflammatory cytokine (TNF-α, IL-1β, PGE2, and NO) levels in the serum and brain, and relevant protein factors (iNOS and COX-2) in the brain. Furthermore, shRNA PSMC5 upregulated the anti-inflammatory mediators interleukin IL-4 and IL-10 in the serum and brain, and promoted a pro-inflammation-to-anti-inflammation phenotype shift in microglial polarization. Mechanistically, shRNA PSMC5 significantly alleviated LPS-induced TLR4 expression. The polarization of LPS-induced microglial pro-inflammation phenotype was abolished by TLR4 inhibitor and in the TLR-4-/- mouse, as in shRNA PSMC5 treatment. PSMC5 interacted with TLR4 via the amino sites Glu284, Met139, Leu127, and Phe283. PSMC5 site mutations attenuated neuroinflammation and reduced pro-inflammatory factors by reducing TLR4-related effects, thereby reducing TLR4-mediated MyD88 (myeloid differentiation factor 88)-dependent activation of NF-κB. PSMC5 could be an important therapeutic target for treatment of neurodegenerative diseases involving neuroinflammation-associated cognitive deficits and motor impairments induced by microglial activation.
Keywords: Luteolin; Microglia; Neuroinflammation; Proteasome 26S subunit (PSMC5), ATPase 5; Synapse; TLR4.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
USP8 protects against lipopolysaccharide-induced cognitive and motor deficits by modulating microglia phenotypes through TLR4/MyD88/NF-κB signaling pathway in mice.Brain Behav Immun. 2020 Aug;88:582-596. doi: 10.1016/j.bbi.2020.04.052. Epub 2020 Apr 23. Brain Behav Immun. 2020. PMID: 32335193
-
Rifampicin ameliorates lipopolysaccharide-induced cognitive and motor impairments via inhibition of the TLR4/MyD88/NF-κB signaling pathway in mice.Neurol Res. 2021 May;43(5):358-371. doi: 10.1080/01616412.2020.1866353. Epub 2021 Mar 21. Neurol Res. 2021. PMID: 33749522
-
Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells.Mol Immunol. 2019 Dec;116:29-37. doi: 10.1016/j.molimm.2019.09.020. Epub 2019 Oct 4. Mol Immunol. 2019. PMID: 31590042
-
Microglia polarization in nociplastic pain: mechanisms and perspectives.Inflammopharmacology. 2023 Jun;31(3):1053-1067. doi: 10.1007/s10787-023-01216-x. Epub 2023 Apr 17. Inflammopharmacology. 2023. PMID: 37069462 Free PMC article. Review.
-
Postoperative analgesia with morphine promoting microglial activation and neuroinflammation induced by surgery aggravates perioperative neurocognitive dysfunction in aged mice.IBRO Neurosci Rep. 2024 Dec 17;18:39-49. doi: 10.1016/j.ibneur.2024.12.008. eCollection 2025 Jun. IBRO Neurosci Rep. 2024. PMID: 39816480 Free PMC article. Review.
Cited by
-
CTRP9 attenuates peripheral nerve injury-induced mechanical allodynia and thermal hyperalgesia through regulating spinal microglial polarization and neuroinflammation mediated by AdipoR1 in male mice.Cell Biol Toxicol. 2024 Oct 26;40(1):91. doi: 10.1007/s10565-024-09933-x. Cell Biol Toxicol. 2024. PMID: 39460844 Free PMC article.
-
Remimazolam attenuated lipopolysaccharide-induced behavioral deficits and neuronal injury via activation of the Nrf2 pathway.Sci Rep. 2025 Apr 21;15(1):13784. doi: 10.1038/s41598-025-95379-y. Sci Rep. 2025. PMID: 40258855 Free PMC article.
-
MFG-E8 Ameliorates Nerve Injury-Induced Neuropathic Pain by Regulating Microglial Polarization and Neuroinflammation via Integrin β3/SOCS3/STAT3 Pathway in Mice.J Neuroimmune Pharmacol. 2024 Sep 21;19(1):49. doi: 10.1007/s11481-024-10150-w. J Neuroimmune Pharmacol. 2024. PMID: 39305375
-
Exploiting Bacterial Effector Proteins to Uncover Evolutionarily Conserved Antiviral Host Machinery.bioRxiv [Preprint]. 2024 Jan 30:2024.01.29.577891. doi: 10.1101/2024.01.29.577891. bioRxiv. 2024. Update in: PLoS Pathog. 2024 May 16;20(5):e1012010. doi: 10.1371/journal.ppat.1012010. PMID: 38352400 Free PMC article. Updated. Preprint.
-
Cannabinoid Receptor-2 Alleviates Sepsis-Induced Neuroinflammation by Modulating Microglia M1/M2 Subset Polarization Through Inhibiting Nogo-B Expression.Mol Neurobiol. 2025 Jul;62(7):9258-9270. doi: 10.1007/s12035-025-04836-2. Epub 2025 Mar 18. Mol Neurobiol. 2025. PMID: 40102346
References
MeSH terms
Substances
Grants and funding
- Nos.2022A1515010842/the Guangdong Basic and Applied Basic Research Foundation
- Nos.202102010099/the Science and Technology Program of Guangzhou
- Nos. SL2023A03J00699/the Science and Technology Program of Guangzhou
- Nos. JNU1AF-CFTP-2022-a01216/the Clinical Frontier Technology Program of the First Affiliated Hospital of Jinan University
- Nos.82071568/the Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

