Synthesis of molecularly imprinted polymer by precipitation polymerization for the removal of ametryn
- PMID: 38001543
- PMCID: PMC10668388
- DOI: 10.1186/s13065-023-01084-0
Synthesis of molecularly imprinted polymer by precipitation polymerization for the removal of ametryn
Abstract
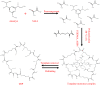
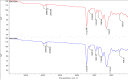
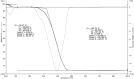
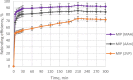
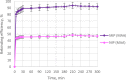
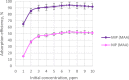
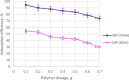
Ametryn (AME) is a triazine herbicide which is mainly used to kill unwanted herbs in crops. Despite its importance in agriculture, the usage of AME also poses a risk to humans and the ecosystem due to its toxicity. Hence, it is important to develop a method for the effective removal of AME from various water sources which is in the form of molecular imprinting polymer (MIP). In this study, MIP of AME was synthesized via precipitation polymerization using AME as the template molecule with three different functional monomers including methacrylic acid (MAA), acrylamide (AAm) and 2-vinylpyridine (2VP). The three different synthesized polymers namely MIP (MAA), MIP (AAm) and MIP (2VP) were characterized using Fourier Infra-red spectroscopy (FTIR) and Field Emission Electron Microscopy (FESEM). Then, the batch binding study was carried out using all three MIPs in which MIP (MAA) attained the highest rebinding efficiency (93.73%) among the synthesized polymers. The Energy-Dispersive X-ray spectroscopy (EDX) analysis, Brunauer-Emmett-Teller (BET) analysis and thermogravimetric analysis (TGA) were also conducted on the selected MIP (MAA). Adsorption studies including initial concentration, pH and polymer dosage were also conducted on MIP (MAA). In this study, the highest adsorption efficiency was attained at the optimum condition of 6 ppm of AME solution at pH 7 with 0.1 g of MIP (MAA). MIP (MAA) was successfully applied to remove AME from spiked distilled water, tap water and river water samples with removal efficiencies of 95.01%, 90.24% and 88.37%, respectively.
Keywords: Ametryn; Herbicide; Molecular imprinted polymer; Precipitation polymerization; Removal; River water; Tap water.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









References
-
- Mesnage R, Szekacs A, Zaller JG. Herbicides: brief history, agricultural use, and potential alternatives for weed control. In: Mesnage R, Zaller JG, editors. Herbicides: chemistry, efficacy, toxicology, and environmental impacts emerging issues in analytical chemistry. 1. Amsterdam: Elsevier; 2021. pp. 1–20.
-
- Bhaskar S, Manoj A, Manu B, Sreenivasa MY, Mudipu V. Non-ferrous Fenton’s oxidation of ametryn using bioleached e-waste copper as catalyst. J Sustain Metall. 2022;8(4):1617–1627. doi: 10.1007/s40831-022-00589-7. - DOI
-
- Hostovsky M, Blahova J, Plhalova L, Kopriva V, Svobodova Z. Effects of the exposure of fish to triazine herbicides. Neuroendocrinol Lett. 2014;35(2):3–25. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
