Evolution of Pharmacological Treatments and Associated Costs for Multiple Myeloma in the Public Healthcare System of Catalonia: A Retrospective Observational Study
- PMID: 38001598
- PMCID: PMC10670024
- DOI: 10.3390/cancers15225338
Evolution of Pharmacological Treatments and Associated Costs for Multiple Myeloma in the Public Healthcare System of Catalonia: A Retrospective Observational Study
Abstract
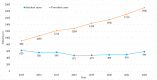
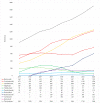
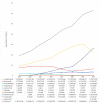
(1) Background: Our understanding of and treatment for multiple myeloma (MM) has advanced significantly, and new pharmacological treatments have promising benefits but high price tags. This study analyzes prescription patterns and pharmaceutical expenditure for MM treatments in Catalonia's public healthcare system over eight years. (2) Methods: A retrospective observational study examined MM treatment data from 2015 to 2022 in Catalonia, using healthcare registries from the Catalan Health Service to collect information on patients, medicines used, and treatment costs. (3) Results: A total of 4556 MM patients received treatment, with a rising trend in the number of treated patients each year from 902 in 2015 to 1899 in 2022. The mean age was 68.9 years, and patients were almost evenly distributed by gender (51.5% male). Most patients were treated with bortezomib (3338 patients), lenalidomide (2952), and/or daratumumab (1093). Most drugs showed increased utilization annually, most significantly for lenalidomide and daratumumab. The total pharmacological treatment cost throughout the entire study period was EUR 321,811,249, with lenalidomide leading with the highest total cost (EUR 157,236,784), and daratumumab exhibiting the highest increase in annual expenditure. (5) Conclusions: The study reveals a progressive increase in the number of MM patients treated and rising pharmaceutical costs. Lenalidomide and daratumumab incurred the highest costs. The findings highlight MM treatment's economic impact and the need to monitor prescription patterns and expenditures to optimize healthcare resources and decision making. Understanding these trends can guide resource allocation effectively.
Keywords: health expenditures; hematological neoplasms; multiple myeloma; pharmacological treatments; treatment patterns.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Use of Drugs in Clinical Practice and the Associated Cost of Cancer Treatment in Adult Patients with Solid Tumors: A 10-Year Retrospective Cohort Study.Curr Oncol. 2023 Aug 30;30(9):7984-8004. doi: 10.3390/curroncol30090580. Curr Oncol. 2023. PMID: 37754495 Free PMC article.
-
Cost-effectiveness of adding daratumumab or bortezomib to lenalidomide plus dexamethasone for newly diagnosed multiple myeloma.J Manag Care Spec Pharm. 2021 Dec;27(12):1691-1702. doi: 10.18553/jmcp.2021.27.12.1691. J Manag Care Spec Pharm. 2021. PMID: 34818089 Free PMC article.
-
Burden of Treatment-Induced Peripheral Neuropathy in Patients with Multiple Myeloma in Sweden.Acta Haematol. 2021;144(5):519-527. doi: 10.1159/000512165. Epub 2021 Feb 25. Acta Haematol. 2021. PMID: 33631745 Free PMC article.
-
Daratumumab for the Management of Newly Diagnosed and Relapsed/Refractory Multiple Myeloma: Current and Emerging Treatments.Front Oncol. 2021 Feb 17;10:624661. doi: 10.3389/fonc.2020.624661. eCollection 2020. Front Oncol. 2021. PMID: 33680948 Free PMC article. Review.
-
Daratumumab for the treatment of multiple myeloma.Expert Opin Biol Ther. 2017 Jul;17(7):887-893. doi: 10.1080/14712598.2017.1322578. Epub 2017 May 2. Expert Opin Biol Ther. 2017. PMID: 28434255 Review.
Cited by
-
The evolving treatment landscape of multiple myeloma in Portugal: A nation-wide retrospective cohort study of real-world clinical practice.EJHaem. 2024 Oct 28;5(6):1144-1153. doi: 10.1002/jha2.1035. eCollection 2024 Dec. EJHaem. 2024. PMID: 39691257 Free PMC article.
-
Health care systems as determinants of outcomes in multiple myeloma: final results from the Latin American MYLACRE study.Blood Adv. 2025 Mar 25;9(6):1293-1302. doi: 10.1182/bloodadvances.2024013838. Blood Adv. 2025. PMID: 39657126 Free PMC article.
References
-
- Dimopoulos M.A., Moreau P., Terpos E., Mateos M.V., Zweegman S., Cook G., Delforge M., Hájek R., Schjesvold F., Cavo M., et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021;32:309–322. doi: 10.1016/j.annonc.2020.11.014. - DOI - PubMed
-
- Dyba T., Randi G., Bray F., Martos C., Giusti F., Nicholson N., Gavin A., Flego M., Neamtiu L., Dimitrova N., et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer. 2021;157:308–347. doi: 10.1016/j.ejca.2021.07.039. - DOI - PMC - PubMed
-
- Palumbo A., Avet-Loiseau H., Oliva S., Lokhorst H.M., Goldschmidt H., Rosinol L., Richardson P., Caltagirone S., Lahuerta J.J., Facon T., et al. Revised international staging system for multiple myeloma: A report from international myeloma working group. J. Clin. Oncol. 2015;33:2863–2869. doi: 10.1200/JCO.2015.61.2267. - DOI - PMC - PubMed