Unveiling a Biomarker Signature of Meningioma: The Need for a Panel of Genomic, Epigenetic, Proteomic, and RNA Biomarkers to Advance Diagnosis and Prognosis
- PMID: 38001599
- PMCID: PMC10670806
- DOI: 10.3390/cancers15225339
Unveiling a Biomarker Signature of Meningioma: The Need for a Panel of Genomic, Epigenetic, Proteomic, and RNA Biomarkers to Advance Diagnosis and Prognosis
Abstract
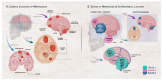
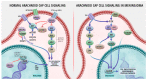
Meningiomas are the most prevalent primary intracranial tumors. The majority are benign but can undergo dedifferentiation into advanced grades classified by World Health Organization (WHO) into Grades 1 to 3. Meningiomas' tremendous variability in tumor behavior and slow growth rates complicate their diagnosis and treatment. A deeper comprehension of the molecular pathways and cellular microenvironment factors implicated in meningioma survival and pathology is needed. This review summarizes the known genetic and epigenetic aberrations involved in meningiomas, with a focus on neurofibromatosis type 2 (NF2) and non-NF2 mutations. Novel potential biomarkers for meningioma diagnosis and prognosis are also discussed, including epigenetic-, RNA-, metabolomics-, and protein-based markers. Finally, the landscape of available meningioma-specific animal models is overviewed. Use of these animal models can enable planning of adjuvant treatment, potentially assisting in pre-operative and post-operative decision making. Discovery of novel biomarkers will allow, in combination with WHO grading, more precise meningioma grading, including meningioma identification, subtype determination, and prediction of metastasis, recurrence, and response to therapy. Moreover, these biomarkers may be exploited in the development of personalized targeted therapies that can distinguish between the 15 diverse meningioma subtypes.
Keywords: NF2 mutations; biomarker; meningioma; miRNA; proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Molecular biomarkers in meningioma (Review).Biomed Rep. 2025 Jan 30;22(4):56. doi: 10.3892/br.2025.1934. eCollection 2025 Apr. Biomed Rep. 2025. PMID: 39991008 Free PMC article. Review.
-
First insight into the somatic mutation burden of neurofibromatosis type 2-associated grade I and grade II meningiomas: a case report comprehensive genomic study of two cranial meningiomas with vastly different clinical presentation.BMC Cancer. 2017 Feb 13;17(1):127. doi: 10.1186/s12885-017-3127-6. BMC Cancer. 2017. PMID: 28193203 Free PMC article.
-
Posttranscriptional deregulation of signaling pathways in meningioma subtypes by differential expression of miRNAs.Neuro Oncol. 2015 Sep;17(9):1250-60. doi: 10.1093/neuonc/nov014. Epub 2015 Feb 13. Neuro Oncol. 2015. PMID: 25681310 Free PMC article.
-
Comprehensive genetic and epigenetic analysis of sporadic meningioma for macro-mutations on 22q and micro-mutations within the NF2 locus.BMC Genomics. 2007 Jan 12;8:16. doi: 10.1186/1471-2164-8-16. BMC Genomics. 2007. PMID: 17222329 Free PMC article.
-
Molecular characteristics of meningiomas.J Pathol Transl Med. 2020 Jan;54(1):45-63. doi: 10.4132/jptm.2019.11.05. Epub 2020 Jan 15. J Pathol Transl Med. 2020. PMID: 31964111 Free PMC article. Review.
Cited by
-
Investigating the Key Trends in Applying Artificial Intelligence to Health Technologies: A Scoping Review.PLoS One. 2025 May 15;20(5):e0322197. doi: 10.1371/journal.pone.0322197. eCollection 2025. PLoS One. 2025. PMID: 40372995 Free PMC article.
-
Potential Causal Relationship Between Plasma and Cerebrospinal Fluid Metabolites and Meningioma: Two-Sample Mendelian Randomization Study.J Multidiscip Healthc. 2025 Jul 31;18:4391-4409. doi: 10.2147/JMDH.S527449. eCollection 2025. J Multidiscip Healthc. 2025. PMID: 40765735 Free PMC article.
-
The Impact of Molecular and Genetic Analysis on the Treatment of Patients with Atypical Meningiomas.Diagnostics (Basel). 2024 Aug 15;14(16):1782. doi: 10.3390/diagnostics14161782. Diagnostics (Basel). 2024. PMID: 39202270 Free PMC article. Review.
-
Molecular biomarkers in meningioma (Review).Biomed Rep. 2025 Jan 30;22(4):56. doi: 10.3892/br.2025.1934. eCollection 2025 Apr. Biomed Rep. 2025. PMID: 39991008 Free PMC article. Review.
-
Biomarkers for prognosis of meningioma patients: A systematic review and meta-analysis.PLoS One. 2024 May 17;19(5):e0303337. doi: 10.1371/journal.pone.0303337. eCollection 2024. PLoS One. 2024. PMID: 38758750 Free PMC article.
References
-
- Gittleman H.R., Ostrom Q.T., Rouse C.D., Dowling J.A., de Blank P.M., Kruchko C.A., Elder J.B., Rosenfeld S.S., Selman W.R., Sloan A.E., et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. 2015;121:102–112. doi: 10.1002/cncr.29015. - DOI - PMC - PubMed
-
- Ostrom Q.T., Gittleman H., Liao P., Vecchione-Koval T., Wolinsky Y., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158. - DOI - PMC - PubMed
-
- Achey R.L., Gittleman H., Schroer J., Khanna V., Kruchko C., Barnholtz-Sloan J.S. Nonmalignant and malignant meningioma incidence and survival in the elderly, 2005–2015, using the Central Brain Tumor Registry of the United States. Neuro-Oncology. 2019;21:380–391. doi: 10.1093/neuonc/noy162. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

