Type 2 Cystatins and Their Roles in the Regulation of Human Immune Response and Cancer Progression
- PMID: 38001623
- PMCID: PMC10670837
- DOI: 10.3390/cancers15225363
Type 2 Cystatins and Their Roles in the Regulation of Human Immune Response and Cancer Progression
Abstract
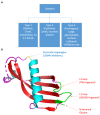
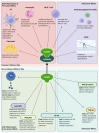
Cystatins are a family of intracellular and extracellular protease inhibitors that inhibit cysteine cathepsins-a group of lysosomal cysteine proteases that participate in multiple biological processes, including protein degradation and post-translational cleavage. Cysteine cathepsins are associated with the development of autoimmune diseases, tumor progression, and metastasis. Cystatins are categorized into three subfamilies: type 1, type 2, and type 3. The type 2 cystatin subfamily is the largest, containing 10 members, and consists entirely of small secreted proteins. Although type 2 cystatins have many shared biological roles, each member differs in structure, post-translational modifications (e.g., glycosylation), and expression in different cell types. These distinctions allow the type 2 cystatins to have unique biological functions and properties. This review provides an overview of type 2 cystatins, including their biological similarities and differences, their regulatory effect on human immune responses, and their roles in tumor progression, immune evasion, and metastasis.
Keywords: cathepsin; cystatin; immunity; inflammation; protease inhibitor; tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cystatins in immune system.J Cancer. 2013;4(1):45-56. doi: 10.7150/jca.5044. Epub 2012 Dec 20. J Cancer. 2013. PMID: 23386904 Free PMC article.
-
Mammalian cystatin and protagonists in brain diseases.J Biomol Struct Dyn. 2020 Apr;38(7):2171-2196. doi: 10.1080/07391102.2019.1620636. Epub 2019 Jun 5. J Biomol Struct Dyn. 2020. PMID: 31107181 Review.
-
Cystatin F as a regulator of immune cell cytotoxicity.Cancer Immunol Immunother. 2018 Dec;67(12):1931-1938. doi: 10.1007/s00262-018-2165-5. Epub 2018 May 10. Cancer Immunol Immunother. 2018. PMID: 29748898 Free PMC article. Review.
-
Cystatins--Extra- and intracellular cysteine protease inhibitors: High-level secretion and uptake of cystatin C in human neuroblastoma cells.Biochimie. 2010 Nov;92(11):1625-34. doi: 10.1016/j.biochi.2010.08.011. Epub 2010 Aug 25. Biochimie. 2010. PMID: 20800088
-
Decreased activity of cathepsins L + B and decreased invasive ability of PC3 prostate cancer cells.Biotech Histochem. 2003 Apr;78(2):101-8. doi: 10.1080/10520290310001593856. Biotech Histochem. 2003. PMID: 14533846
Cited by
-
Positive Role of Saliva in the Oral Microbiome.Adv Exp Med Biol. 2025;1472:103-118. doi: 10.1007/978-3-031-79146-8_7. Adv Exp Med Biol. 2025. PMID: 40111688 Review.
-
Serum cystatin C as a potential biomarker for generalized acetylcholine receptor antibody-positive myasthenia gravis.Front Immunol. 2025 May 8;16:1578359. doi: 10.3389/fimmu.2025.1578359. eCollection 2025. Front Immunol. 2025. PMID: 40406138 Free PMC article.
-
Investigation of CST7 and hsa-miR-4793-5p gene expression in breast cancer.Biochem Biophys Rep. 2024 Nov 1;40:101863. doi: 10.1016/j.bbrep.2024.101863. eCollection 2024 Dec. Biochem Biophys Rep. 2024. PMID: 39552709 Free PMC article.
-
Plasma proteomics and carotid intima-media thickness in the UK biobank cohort.Front Cardiovasc Med. 2024 Oct 2;11:1478600. doi: 10.3389/fcvm.2024.1478600. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39416432 Free PMC article.
-
Association of mental disorders with sepsis: a bidirectional Mendelian randomization study.Front Public Health. 2024 May 17;12:1327315. doi: 10.3389/fpubh.2024.1327315. eCollection 2024. Front Public Health. 2024. PMID: 38827616 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

