Oleic Acid Exhibits Anti-Proliferative and Anti-Invasive Activities via the PTEN/AKT/mTOR Pathway in Endometrial Cancer
- PMID: 38001668
- PMCID: PMC10670880
- DOI: 10.3390/cancers15225407
Oleic Acid Exhibits Anti-Proliferative and Anti-Invasive Activities via the PTEN/AKT/mTOR Pathway in Endometrial Cancer
Abstract
Reprogramming of fatty acid metabolism promotes cell growth and metastasis through a variety of processes that stimulate signaling molecules, energy storage, and membrane biosynthesis in endometrial cancer. Oleic acid is one of the most important monounsaturated fatty acids in the human body, which appears to have both pro- and anti-tumorigenic activities in various pre-clinical models. In this study, we evaluated the potential anti-tumor effects of oleic acid in endometrial cancer cells and the LKB1fl/flp53fl/fl mouse model of endometrial cancer. Oleic acid increased lipogenesis, inhibited cell proliferation, caused cell cycle G1 arrest, induced cellular stress and apoptosis, and suppressed invasion in endometrial cancer cells. Targeting of diacylglycerol acyltransferases 1 and 2 effectively increased the cytotoxicity of oleic acid. Moreover, oleic acid significantly increased the expression of wild-type PTEN, and knockdown of PTEN by shRNA partially reversed the anti-proliferative and anti-invasive effects of oleic acid. Inhibition of the AKT/mTOR pathway by ipatasertib effectively increased the anti-tumor activity of oleic acid in endometrial cancer cells. Oleic acid treatment (10 mg/kg, daily, oral) for four weeks significantly inhibited tumor growth by 52.1% in the LKB1fl/flp53fl/fl mice. Our findings demonstrated that oleic acid exhibited anti-tumorigenic activities, dependent on the PTEN/AKT/mTOR signaling pathway, in endometrial cancer.
Keywords: PTEN/AKT pathway; cell proliferation; endometrial cancer; invasion; lipid droplets; oleic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
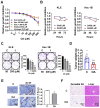
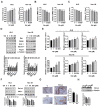

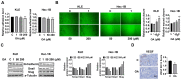




Similar articles
-
High-dose Ascorbate Exhibits Anti-proliferative and Anti-invasive Effects Dependent on PTEN/AKT/mTOR Pathway in Endometrial Cancer in vitro and in vivo.Int J Biol Sci. 2025 Jan 27;21(4):1545-1565. doi: 10.7150/ijbs.102079. eCollection 2025. Int J Biol Sci. 2025. PMID: 39990670 Free PMC article.
-
Linoleic acid exhibits anti-proliferative and anti-invasive activities in endometrial cancer cells and a transgenic model of endometrial cancer.Cancer Biol Ther. 2024 Dec 31;25(1):2325130. doi: 10.1080/15384047.2024.2325130. Epub 2024 Mar 11. Cancer Biol Ther. 2024. PMID: 38465855 Free PMC article.
-
Onvansertib exhibits anti-proliferative and anti-invasive effects in endometrial cancer.Front Pharmacol. 2025 Mar 17;16:1545038. doi: 10.3389/fphar.2025.1545038. eCollection 2025. Front Pharmacol. 2025. PMID: 40166466 Free PMC article.
-
Targeting dopamine receptor D2 as a novel therapeutic strategy in endometrial cancer.J Exp Clin Cancer Res. 2021 Feb 8;40(1):61. doi: 10.1186/s13046-021-01842-9. J Exp Clin Cancer Res. 2021. PMID: 33557912 Free PMC article.
-
Chemoresistance and targeting of growth factors/cytokines signalling pathways: towards the development of effective therapeutic strategy for endometrial cancer.Am J Cancer Res. 2018 Jul 1;8(7):1317-1331. eCollection 2018. Am J Cancer Res. 2018. PMID: 30094104 Free PMC article. Review.
Cited by
-
Phosphocholine inhibits proliferation and reduces stemness of endometrial cancer cells by downregulating mTOR-c-Myc signaling.Cell Mol Life Sci. 2024 Dec 16;82(1):3. doi: 10.1007/s00018-024-05517-4. Cell Mol Life Sci. 2024. PMID: 39680126 Free PMC article.
-
MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro.Open Life Sci. 2024 Dec 31;19(1):20220990. doi: 10.1515/biol-2022-0990. eCollection 2024. Open Life Sci. 2024. PMID: 39759103 Free PMC article.
-
Folic Acid-Conjugated Magnetic Oleoyl-Chitosan Nanoparticles for Controlled Release of Doxorubicin in Cancer Therapy.Nanomaterials (Basel). 2025 Mar 7;15(6):415. doi: 10.3390/nano15060415. Nanomaterials (Basel). 2025. PMID: 40137588 Free PMC article.
-
Diagnostic Value of Menstrual Blood Lipidomics in Endometriosis: A Pilot Study.Biomolecules. 2024 Jul 24;14(8):899. doi: 10.3390/biom14080899. Biomolecules. 2024. PMID: 39199287 Free PMC article.
-
Antioxidant, cytotoxic, anti-migratory, and pro-apoptotic effects of Bolanthus turcicus extracts on head and neck cancer cells.Mol Biol Rep. 2024 Oct 30;51(1):1104. doi: 10.1007/s11033-024-09994-5. Mol Biol Rep. 2024. PMID: 39476042
References
-
- Colombo N., Creutzberg C., Amant F., Bosse T., Gonzalez-Martin A., Ledermann J., Marth C., Nout R., Querleu D., Mirza M.R., et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016;27:16–41. doi: 10.1093/annonc/mdv484. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

