STAT2 Controls Colorectal Tumorigenesis and Resistance to Anti-Cancer Drugs
- PMID: 38001683
- PMCID: PMC10670206
- DOI: 10.3390/cancers15225423
STAT2 Controls Colorectal Tumorigenesis and Resistance to Anti-Cancer Drugs
Abstract
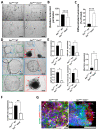
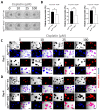
Colorectal cancer (CRC) is a significant socioeconomic burden in modern society and is accountable for millions of premature deaths each year. The role of signal transducer and activator of transcription 2 (STAT2)-dependent signaling in this context is not yet fully understood, and no therapies targeting this pathway are currently being pursued. We investigated the role of STAT2 in CRC using experimental mouse models coupled with RNA-sequencing (RNA-Seq) data and functional assays with anti-cancer agents in three-dimensional tumoroids. Stat2-/- mice showed greater resistance to the development of CRC in both inflammation-driven and inflammation-independent experimental CRC models. In ex vivo studies, tumoroids derived from Stat2-/- mice with the multiple intestinal neoplasia (Min) mutant allele of the adenomatous polyposis coli (Apc) locus exhibited delayed growth, were overall smaller and more differentiated as compared with tumoroids from ApcMin/+ wildtype (WT) mice. Notably, tumoroids from ApcMin/+ Stat2-/- mice were more susceptible to anti-cancer agents inducing cell death by different mechanisms. Our findings clearly indicated that STAT2 promotes CRC and suggested that interventions targeting STAT2-dependent signals might become an attractive therapeutic option for patients with CRC.
Keywords: ApcMin/+; STAT2; anti-cancer therapy; colorectal cancer mouse model; tumoroid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer.Mol Cancer. 2018 Jan 5;17(1):1. doi: 10.1186/s12943-017-0753-1. Mol Cancer. 2018. PMID: 29304823 Free PMC article.
-
TRIB3 Interacts With β-Catenin and TCF4 to Increase Stem Cell Features of Colorectal Cancer Stem Cells and Tumorigenesis.Gastroenterology. 2019 Feb;156(3):708-721.e15. doi: 10.1053/j.gastro.2018.10.031. Epub 2018 Oct 24. Gastroenterology. 2019. PMID: 30365932
-
Nrf2 knockout enhances intestinal tumorigenesis in Apc(min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation.Mol Carcinog. 2014 Jan;53(1):77-84. doi: 10.1002/mc.21950. Epub 2012 Aug 21. Mol Carcinog. 2014. PMID: 22911891
-
Signal transduction cross-talk during colorectal tumorigenesis.Adv Anat Pathol. 2006 Sep;13(5):270-4. doi: 10.1097/01.pap.0000213046.61941.5c. Adv Anat Pathol. 2006. PMID: 16998321 Review.
-
Interaction of the Wnt/β-catenin and RAS-ERK pathways involving co-stabilization of both β-catenin and RAS plays important roles in the colorectal tumorigenesis.Adv Biol Regul. 2018 May;68:46-54. doi: 10.1016/j.jbior.2018.01.001. Epub 2018 Jan 10. Adv Biol Regul. 2018. PMID: 29449169 Review.
Cited by
-
ISG15 promotes tumor progression via IL6/JAK2/STAT3 signaling pathway in ccRCC.Clin Exp Med. 2024 Jun 29;24(1):140. doi: 10.1007/s10238-024-01414-z. Clin Exp Med. 2024. PMID: 38951255 Free PMC article.
-
Single-Cell RNA Sequencing Reveals Extensive Heterogeneity and Unique Gene Trajectories in Non-Transformed and Transformed Human Lung Epithelial Cells: Insights into the Role of LncRNAs in Tumor Heterogeneity.Int J Mol Sci. 2025 Feb 16;26(4):1690. doi: 10.3390/ijms26041690. Int J Mol Sci. 2025. PMID: 40004153 Free PMC article.
-
Tissue-specific atlas of trans-models for gene regulation elucidates complex regulation patterns.BMC Genomics. 2024 Apr 17;25(1):377. doi: 10.1186/s12864-024-10317-y. BMC Genomics. 2024. PMID: 38632500 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

