The Diversity of Liquid Biopsies and Their Potential in Breast Cancer Management
- PMID: 38001722
- PMCID: PMC10670968
- DOI: 10.3390/cancers15225463
The Diversity of Liquid Biopsies and Their Potential in Breast Cancer Management
Abstract
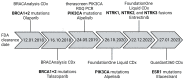
Analyzing blood as a so-called liquid biopsy in breast cancer (BC) patients has the potential to adapt therapy management. Circulating tumor cells (CTCs), extracellular vesicles (EVs), cell-free DNA (cfDNA) and other blood components mirror the tumoral heterogeneity and could support a range of clinical decisions. Multi-cancer early detection tests utilizing blood are advancing but are not part of any clinical routine yet. Liquid biopsy analysis in the course of neoadjuvant therapy has potential for therapy (de)escalation.Minimal residual disease detection via serial cfDNA analysis is currently on its way. The prognostic value of blood analytes in early and metastatic BC is undisputable, but the value of these prognostic biomarkers for clinical management is controversial. An interventional trial confirmed a significant outcome benefit when therapy was changed in case of newly emerging cfDNA mutations under treatment and thus showed the clinical utility of cfDNA analysis for therapy monitoring. The analysis of PIK3CA or ESR1 variants in plasma of metastatic BC patients to prescribe targeted therapy with alpesilib or elacestrant has already arrived in clinical practice with FDA-approved tests available and is recommended by ASCO. The translation of more liquid biopsy applications into clinical practice is still pending due to a lack of knowledge of the analytes' biology, lack of standards and difficulties in proving clinical utility.
Keywords: blood; breast neoplasm; drug response biomarkers; early detection of cancer; genetic predictive testing; liquid biopsy; precision medicine; prognosis; residual neoplasm.
Conflict of interest statement
C.K. received support for travel expenses from QIAGEN, Hilden, Germany. R.K. has received honoraria from Tesaro, MSD, GSK and Astra-Zeneca in the last 3 years, is part of the advisory board of Medtronic and council of IGCS and President of SERGS and has proctored and presented for Intuitive Surgical. S.K.-B. is a consultant for QIAGEN, Hilden, Germany.
Figures

Similar articles
-
Diagnostic utility of ESR1 mutation detection in liquid biopsy of metastatic breast cancer patients.Virchows Arch. 2024 Oct 11. doi: 10.1007/s00428-024-03942-1. Online ahead of print. Virchows Arch. 2024. PMID: 39392509
-
Longitudinal Multi-Parametric Liquid Biopsy Approach Identifies Unique Features of Circulating Tumor Cell, Extracellular Vesicle, and Cell-Free DNA Characterization for Disease Monitoring in Metastatic Breast Cancer Patients.Cells. 2021 Jan 21;10(2):212. doi: 10.3390/cells10020212. Cells. 2021. PMID: 33494385 Free PMC article.
-
Translation of Epigenetics in Cell-Free DNA Liquid Biopsy Technology and Precision Oncology.Curr Issues Mol Biol. 2024 Jun 27;46(7):6533-6565. doi: 10.3390/cimb46070390. Curr Issues Mol Biol. 2024. PMID: 39057032 Free PMC article. Review.
-
Liquid biopsy and its role in an advanced clinical trial for lung cancer.Exp Biol Med (Maywood). 2018 Feb;243(3):262-271. doi: 10.1177/1535370217750087. Exp Biol Med (Maywood). 2018. PMID: 29405770 Free PMC article. Review.
-
Liquid biopsy: current technology and clinical applications.J Hematol Oncol. 2022 Sep 12;15(1):131. doi: 10.1186/s13045-022-01351-y. J Hematol Oncol. 2022. PMID: 36096847 Free PMC article. Review.
Cited by
-
Circulating tumor cells et al.: towards a comprehensive liquid biopsy approach in breast cancer.Transl Breast Cancer Res. 2024 Apr 26;5:10. doi: 10.21037/tbcr-23-55. eCollection 2024. Transl Breast Cancer Res. 2024. PMID: 38751670 Free PMC article. Review.
-
The role of pioneering transcription factors, chromatin accessibility and epigenetic reprogramming in oncogenic viruses.Front Microbiol. 2025 Jun 16;16:1602497. doi: 10.3389/fmicb.2025.1602497. eCollection 2025. Front Microbiol. 2025. PMID: 40589575 Free PMC article. Review.
-
Comprehensive review of male breast cancer: Understanding a rare condition.Oncol Res. 2025 May 29;33(6):1289-1300. doi: 10.32604/or.2025.058790. eCollection 2025. Oncol Res. 2025. PMID: 40486874 Free PMC article. Review.
-
The impact of liquid biopsy in breast cancer: Redefining the landscape of non-invasive precision oncology.J Liq Biopsy. 2025 May 21;8:100299. doi: 10.1016/j.jlb.2025.100299. eCollection 2025 Jun. J Liq Biopsy. 2025. PMID: 40521566 Free PMC article. Review.
-
Male Breast Cancer: Current Scenario and Future Perspectives.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241261836. doi: 10.1177/15330338241261836. Technol Cancer Res Treat. 2024. PMID: 39043043 Free PMC article. Review.
References
-
- Wolff A.C., Somerfield M.R., Dowsett M., Hammond M.E.H., Hayes D.F., McShane L.M., Saphner T.J., Spears P.A., Allison K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 2023;41:3867–3872. doi: 10.1200/JCO.22.02864. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

