CX3CR1-Expressing Immune Cells Infiltrate the Tumor Microenvironment and Promote Radiation Resistance in a Mouse Model of Lung Cancer
- PMID: 38001732
- PMCID: PMC10669975
- DOI: 10.3390/cancers15225472
CX3CR1-Expressing Immune Cells Infiltrate the Tumor Microenvironment and Promote Radiation Resistance in a Mouse Model of Lung Cancer
Abstract
Introduction: Chemokine (C-X3-C Motif) Receptor 1 (CX3CR1) is present in a subset of the immune cells in the tumor microenvironment (TME) and plays an essential and diverse role in cancer progression. However, its potential function in the irradiated TME remains unknown.
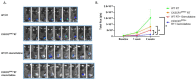
Materials and methods: A mouse lung cancer model was performed by subcutaneously inoculating Lewis Lung Carcinoma (LLC) cells expressing luciferase (Luc-2) and mCherry cells in CX3CR1GFP/GFP, CX3CR1DTR/+, and wild-type (WT) mice. Bioluminescence imaging, clonogenic assay, and flow cytometry were used to assess tumor progression, proliferation, and cell composition after radiation.
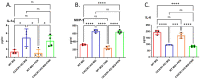
Results: Radiation provoked a significant influx of CX3CR1-expressing immune cells, notably monocytes and macrophages, into the TME. Co-culturing irradiated LLC cells with CX3CR1-deficient monocytes, and macrophages resulted in reduced clonogenic survival and increased apoptosis of the cancer cells. Interestingly, deficiency of CX3CR1 in macrophages led to a redistribution of the irradiated LLC cells in the S-phase, parallel to increased expression of cyclin E1, required for cell cycle G1/S transition. In addition, the deficiency of CX3CR1 expression in macrophages altered the cytokine secretion with a decrease in interleukin 6, a crucial mediator of cancer cell survival and proliferation. Next, LLC cells were injected subcutaneously into CX3CR1DTR/+ mice, sensitive to diphtheria toxin (DT), and WT mice. After injection, tumors were irradiated with 8 Gy, and mice were treated with DT, leading to conditional ablation of CX3CR1-expressing cells. After three weeks, CX3CR1-depleted mice displayed reduced tumor progression. Furthermore, combining the S-phase-specific chemotherapeutic gemcitabine with CX3CR1 cell ablation resulted in additional attenuation of tumor progression.
Conclusions: CX3CR1-expressing mononuclear cells invade the TME after radiation therapy in a mouse lung cancer model. CX3CR1 cell depletion attenuates tumor progression following radiation and sensitizes the tumor to S-phase-specific chemotherapy. Thus, we propose a novel strategy to improve radiation sensitivity by targeting the CX3CR1-expressing immune cells.
Keywords: cytokines; immunotherapy; lung cancer; radiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Blocking LTB4 signaling-mediated TAMs recruitment by Rhizoma Coptidis sensitizes lung cancer to immunotherapy.Phytomedicine. 2023 Oct;119:154968. doi: 10.1016/j.phymed.2023.154968. Epub 2023 Jul 22. Phytomedicine. 2023. PMID: 37531900
-
Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer.Am J Respir Crit Care Med. 2015 Feb 15;191(4):437-47. doi: 10.1164/rccm.201406-1137OC. Am J Respir Crit Care Med. 2015. PMID: 25536148
-
CX3CR1 deficiency exacerbates immune-mediated hepatitis by increasing NF-κB-mediated cytokine production in macrophage and T cell.Exp Biol Med (Maywood). 2023 Jan;248(2):117-129. doi: 10.1177/15353702221128573. Epub 2022 Nov 25. Exp Biol Med (Maywood). 2023. PMID: 36426712 Free PMC article.
-
In vivo two-photon characterization of tumor-associated macrophages and microglia (TAM/M) and CX3CR1 during different steps of brain metastasis formation from lung cancer.Neoplasia. 2021 Nov;23(11):1089-1100. doi: 10.1016/j.neo.2021.09.001. Epub 2021 Sep 26. Neoplasia. 2021. PMID: 34587566 Free PMC article.
-
Tissue-specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease.Immune Netw. 2018 Jan 25;18(1):e5. doi: 10.4110/in.2018.18.e5. eCollection 2018 Feb. Immune Netw. 2018. PMID: 29503738 Free PMC article. Review.
Cited by
-
Exploring CX3CR1 as a prognostic biomarker and immunotherapeutic target in sarcoma.Transl Oncol. 2025 Mar;53:102283. doi: 10.1016/j.tranon.2025.102283. Epub 2025 Jan 20. Transl Oncol. 2025. PMID: 39837057 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

