The Contribution of Hippocampal All-Trans Retinoic Acid (ATRA) Deficiency to Alzheimer's Disease: A Narrative Overview of ATRA-Dependent Gene Expression in Post-Mortem Hippocampal Tissue
- PMID: 38001775
- PMCID: PMC10669734
- DOI: 10.3390/antiox12111921
The Contribution of Hippocampal All-Trans Retinoic Acid (ATRA) Deficiency to Alzheimer's Disease: A Narrative Overview of ATRA-Dependent Gene Expression in Post-Mortem Hippocampal Tissue
Abstract
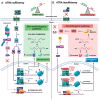
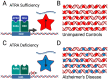
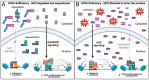
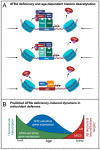
There is accumulating evidence that vitamin A (VA) deficiency contributes to the pathogenesis and progression of Alzheimer's disease (AD). All-trans retinoic acid (ATRA), a metabolite of VA in the brain, serves distinct roles in the human hippocampus. Agonists of retinoic acid receptors (RAR), including ATRA, promote activation of the non-amyloidogenic pathway by enhancing expression of α-secretases, providing a mechanistic basis for delaying/preventing amyloid beta (Aβ) toxicity. However, whether ATRA is actually deficient in the hippocampi of patients with AD is not clear. Here, using a publicly available human transcriptomic dataset, we evaluated the extent to which ATRA-sensitive genes are dysregulated in hippocampal tissue from post-mortem AD brains, relative to age-matched controls. Consistent with ATRA deficiency, we found significant dysregulation of many ATRA-sensitive genes and significant upregulation of RAR co-repressors, supporting the idea of transcriptional repression of ATRA-mediated signaling. Consistent with oxidative stress and neuroinflammation, Nrf2 and NfkB transcripts were upregulated, respectively. Interestingly, transcriptional targets of Nrf2 were not upregulated, accompanied by upregulation of several histone deacetylases. Overall, our investigation of ATRA-sensitive genes in the human hippocampus bolsters the scientific premise of ATRA depletion in AD and that epigenetic factors should be considered and addressed as part of VA supplementation.
Keywords: Alzheimer’s disease; aging; retinoic acid; vitamin A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Correction of all-trans retinoic acid deficiency in alcoholic cirrhosis lessens the excessive inflammatory monocyte response: a translational study.Liver Int. 2014 Mar;34(3):343-52. doi: 10.1111/liv.12249. Epub 2013 Jul 8. Liver Int. 2014. PMID: 23834309
-
Retinol inhibits the growth of all-trans-retinoic acid-sensitive and all-trans-retinoic acid-resistant colon cancer cells through a retinoic acid receptor-independent mechanism.Cancer Res. 2005 Nov 1;65(21):9923-33. doi: 10.1158/0008-5472.CAN-05-1604. Cancer Res. 2005. PMID: 16267017
-
All-trans-retinoic acid reduces BACE1 expression under inflammatory conditions via modulation of nuclear factor κB (NFκB) signaling.J Biol Chem. 2015 Sep 11;290(37):22532-42. doi: 10.1074/jbc.M115.662908. Epub 2015 Aug 3. J Biol Chem. 2015. PMID: 26240147 Free PMC article.
-
ATRA(ouble) in the treatment of acute promyelocytic leukemia.J Biol Regul Homeost Agents. 2001 Apr-Jun;15(2):107-22. J Biol Regul Homeost Agents. 2001. PMID: 11501968 Review.
-
All-trans Retinoic Acid as a Versatile Cytosolic Signal Modulator Mediated by CRABP1.Int J Mol Sci. 2019 Jul 24;20(15):3610. doi: 10.3390/ijms20153610. Int J Mol Sci. 2019. PMID: 31344789 Free PMC article. Review.
Cited by
-
Beneficial Effects of Manilkara zapota-Derived Bioactive Compounds in the Epigenetic Program of Neurodevelopment.Nutrients. 2024 Jul 11;16(14):2225. doi: 10.3390/nu16142225. Nutrients. 2024. PMID: 39064669 Free PMC article. Review.
-
Low-dose dietary vorinostat increases brain histone acetylation levels and reduces oxidative stress in an Alzheimer's disease mouse model.J Alzheimers Dis. 2025 Aug;106(4):1360-1382. doi: 10.1177/13872877251352107. Epub 2025 Jul 1. J Alzheimers Dis. 2025. PMID: 40598871 Free PMC article.
-
A Comparative Study of a Potent CNS-Permeable RARβ-Modulator, Ellorarxine, in Neurons, Glia and Microglia Cells In Vitro.Int J Mol Sci. 2025 Apr 10;26(8):3551. doi: 10.3390/ijms26083551. Int J Mol Sci. 2025. PMID: 40332055 Free PMC article.
-
Key target genes related to anti-breast cancer activity of ATRA: A network pharmacology, molecular docking and experimental investigation.Heliyon. 2024 Jul 9;10(14):e34300. doi: 10.1016/j.heliyon.2024.e34300. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39108872 Free PMC article.
References
-
- Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., Amann M., Anderson H.R., Andrews K.G., Aryee M., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. - DOI - PMC - PubMed
-
- Lamar M., Boots E.A., Arfanakis K., Barnes L.L., Schneider J.A. Common Brain Structural Alterations Associated with Cardiovascular Disease Risk Factors and Alzheimer’s Dementia: Future Directions and Implications. Neuropsychol. Rev. 2020;30:546–557. doi: 10.1007/s11065-020-09460-6. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

