Korean Chestnut Honey Suppresses HSV-1 Infection by Regulating the ROS-NLRP3 Inflammasome Pathway
- PMID: 38001788
- PMCID: PMC10669648
- DOI: 10.3390/antiox12111935
Korean Chestnut Honey Suppresses HSV-1 Infection by Regulating the ROS-NLRP3 Inflammasome Pathway
Abstract
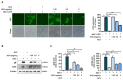
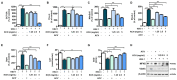
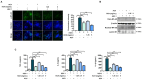
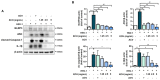
Herpes simplex virus 1 (HSV-1) is double-stranded DNA virus that belongs to the Orthoherpesviridae family. It causes serious neurological diseases of the central nervous system, such as encephalitis. The current U.S. Food and Drug Administration (FDA)-approved drugs for preventing HSV-1 infection include acyclovir (ACV) and valacyclovir; however, their long-term use causes severe side effects and often results in the emergence of drug-resistant strains. Therefore, it is important to discover new antiviral agents that are safe and effective against HSV-1 infection. Korean chestnut honey (KCH) has various pharmacological activities, such as antioxidant, antibacterial, and anti-inflammation effects; however, antiviral effects against HSV-1 have not yet been reported. Therefore, we determined the antiviral activity and mechanism of action of KCH after HSV-1 infection on the cellular level. KCH inhibited the HSV-1 infection of host cells through binding and virucidal steps. KCH decreased the production of reactive oxygen species (ROS) and calcium (Ca2+) following HSV-1 infection and suppressed the production of inflammatory cytokines by inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-кB) activity. Furthermore, we found that KCH inhibited the expression of the nod-like receptor protein 3 (NLRP3) inflammasome during HSV-1 infection. Taken together, the antiviral effects of KCH occur through multiple targets, including the inhibition of viral replication and the ROS-mediated NLRP3 inflammasome pathway. Our findings suggest that KCH has potential for the treatment of HSV-1 infection and related diseases.
Keywords: HSV-1; KCH; NF-кB; NLRP3 inflammasome; ROS; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Quercus acuta Thunb. (Fagaceae) and Its Component, Isoquercitrin, Inhibit HSV-1 Replication by Suppressing Virus-Induced ROS Production and NF-κB Activation.Antioxidants (Basel). 2021 Oct 18;10(10):1638. doi: 10.3390/antiox10101638. Antioxidants (Basel). 2021. PMID: 34679772 Free PMC article.
-
Antiviral Activity of Oridonin Against Herpes Simplex Virus Type 1.Drug Des Devel Ther. 2022 Dec 20;16:4311-4323. doi: 10.2147/DDDT.S387885. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36573068 Free PMC article.
-
Boswellia serrata oleo-gum-resin and β-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-кB and p38 MAP kinase signaling.Phytomedicine. 2018 Dec 1;51:94-103. doi: 10.1016/j.phymed.2018.10.016. Epub 2018 Oct 16. Phytomedicine. 2018. PMID: 30466633
-
[Herpes simplex virus latency, reactivation, and a new antiviral therapy for herpetic keratitis].Nippon Ganka Gakkai Zasshi. 2008 Mar;112(3):247-64; discussion 265. Nippon Ganka Gakkai Zasshi. 2008. PMID: 18411713 Review. Japanese.
-
Herpes simplex virus infection.Semin Pediatr Infect Dis. 2002 Jan;13(1):6-11. doi: 10.1053/spid.2002.29752. Semin Pediatr Infect Dis. 2002. PMID: 12118847 Review.
Cited by
-
Antimicrobial Potential of Bee-Derived Products: Insights into Honey, Propolis and Bee Venom.Pathogens. 2025 Aug 6;14(8):780. doi: 10.3390/pathogens14080780. Pathogens. 2025. PMID: 40872290 Free PMC article. Review.
-
Anti-HSV-1 agents: an update.Front Pharmacol. 2025 Jan 21;15:1451083. doi: 10.3389/fphar.2024.1451083. eCollection 2024. Front Pharmacol. 2025. PMID: 39931518 Free PMC article. Review.
-
HSV-1 hijacks mitochondrial dynamics: potential molecular mechanisms linking viral infection to neurodegenerative disorders.Apoptosis. 2025 Jul 1. doi: 10.1007/s10495-025-02142-9. Online ahead of print. Apoptosis. 2025. PMID: 40593393 Review.
-
JAG1 mediates apoptosis in herpes simplex keratitis by suppressing autophagy via ROS/JAG1/NOTCH1/pULK1 signaling pathway.Cell Biol Toxicol. 2024 Dec 20;41(1):1. doi: 10.1007/s10565-024-09968-0. Cell Biol Toxicol. 2024. PMID: 39704867 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

