The Key Role of GSH in Keeping the Redox Balance in Mammalian Cells: Mechanisms and Significance of GSH in Detoxification via Formation of Conjugates
- PMID: 38001806
- PMCID: PMC10669396
- DOI: 10.3390/antiox12111953
The Key Role of GSH in Keeping the Redox Balance in Mammalian Cells: Mechanisms and Significance of GSH in Detoxification via Formation of Conjugates
Abstract
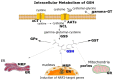
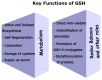
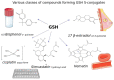
Glutathione (GSH) is a ubiquitous tripeptide that is biosynthesized in situ at high concentrations (1-5 mM) and involved in the regulation of cellular homeostasis via multiple mechanisms. The main known action of GSH is its antioxidant capacity, which aids in maintaining the redox cycle of cells. To this end, GSH peroxidases contribute to the scavenging of various forms of ROS and RNS. A generally underestimated mechanism of action of GSH is its direct nucleophilic interaction with electrophilic compounds yielding thioether GSH S-conjugates. Many compounds, including xenobiotics (such as NAPQI, simvastatin, cisplatin, and barbital) and intrinsic compounds (such as menadione, leukotrienes, prostaglandins, and dopamine), form covalent adducts with GSH leading mainly to their detoxification. In the present article, we wish to present the key role and significance of GSH in cellular redox biology. This includes an update on the formation of GSH-S conjugates or GSH adducts with emphasis given to the mechanism of reaction, the dependence on GST (GSH S-transferase), where this conjugation occurs in tissues, and its significance. The uncovering of the GSH adducts' formation enhances our knowledge of the human metabolome. GSH-hematin adducts were recently shown to have been formed spontaneously in multiples isomers at hemolysates, leading to structural destabilization of the endogenous toxin, hematin (free heme), which is derived from the released hemoglobin. Moreover, hemin (the form of oxidized heme) has been found to act through the Kelch-like ECH associated protein 1 (Keap1)-nuclear factor erythroid 2-related factor-2 (Nrf2) signaling pathway as an epigenetic modulator of GSH metabolism. Last but not least, the implications of the genetic defects in GSH metabolism, recorded in hemolytic syndromes, cancer and other pathologies, are presented and discussed under the framework of conceptualizing that GSH S-conjugates could be regarded as signatures of the cellular metabolism in the diseased state.
Keywords: GSH S-conjugates; GSH adducts; GSH metabolism; GSH redox cycle; GSH-hematin adducts; GSTs; hemolytic disorders; metabolic signatures; thioether bonds; xenobiotics.
Conflict of interest statement
The authors declare have no conflict of interest.
Figures
Similar articles
-
Glutathione-Hemin/Hematin Adduct Formation to Disintegrate Cytotoxic Oxidant Hemin/Hematin in Human K562 Cells and Red Blood Cells' Hemolysates: Impact of Glutathione on the Hemolytic Disorders and Homeostasis.Antioxidants (Basel). 2022 Sep 30;11(10):1959. doi: 10.3390/antiox11101959. Antioxidants (Basel). 2022. PMID: 36290682 Free PMC article.
-
Reprint of: Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs.Free Radic Biol Med. 2014 Jan;66:45-57. doi: 10.1016/j.freeradbiomed.2013.11.002. Epub 2013 Nov 18. Free Radic Biol Med. 2014. PMID: 24262357
-
Endogenous glutathione adducts.Curr Drug Metab. 2006 Dec;7(8):853-72. doi: 10.2174/138920006779010601. Curr Drug Metab. 2006. PMID: 17168687 Review.
-
Activation of KEAP1/NRF2 stress signaling involved in the molecular basis of hemin-induced cytotoxicity in human pro-erythroid K562 cells.Biochem Pharmacol. 2020 May;175:113900. doi: 10.1016/j.bcp.2020.113900. Epub 2020 Mar 7. Biochem Pharmacol. 2020. PMID: 32156661
-
Redox Regulation of Xenobiotics by Reactive Sulfur and Supersulfide Species.Antioxid Redox Signal. 2024 Apr;40(10-12):679-690. doi: 10.1089/ars.2022.0172. Epub 2023 Sep 5. Antioxid Redox Signal. 2024. PMID: 37294201 Review.
Cited by
-
QingGan LiDan capsules improved alcoholic liver injury by regulating liver lipid transport and oxidative stress in mice.Front Pharmacol. 2025 Mar 26;16:1575280. doi: 10.3389/fphar.2025.1575280. eCollection 2025. Front Pharmacol. 2025. PMID: 40206071 Free PMC article.
-
Bioactive compounds in Raphanus sativus: mechanisms of apoptosis, anti-angiogenesis, cell cycle arrest and beyond in cancer prevention and treatment.Med Oncol. 2025 Jul 13;42(8):328. doi: 10.1007/s12032-025-02894-z. Med Oncol. 2025. PMID: 40652415 Review.
-
Alterations in GSH/GSSG and CyS/CySS redox status in small cell lung cancer patients undergoing chemotherapy.Discov Oncol. 2025 Jul 30;16(1):1445. doi: 10.1007/s12672-025-03251-2. Discov Oncol. 2025. PMID: 40739043 Free PMC article.
-
The Development and Assessment of a Unique Disulfidptosis-Associated lncRNA Profile for Immune Microenvironment Prediction and Personalized Therapy in Gastric Adenocarcinoma.Biomedicines. 2025 May 19;13(5):1224. doi: 10.3390/biomedicines13051224. Biomedicines. 2025. PMID: 40427051 Free PMC article.
-
Panose prevents acute-on-chronic liver failure by reducing bacterial infection in mice.J Clin Invest. 2025 Jun 3;135(14):e184653. doi: 10.1172/JCI184653. eCollection 2025 Jul 15. J Clin Invest. 2025. PMID: 40478727 Free PMC article.
References
-
- Rey-Pailhade D. Sur un corps d’origine organique hydrogénant le soufre á froid. Hebd. Séances Acad. Sci. 1888;106:1683–1684.
-
- Rey-Pailhade D. Sur un nouveau principe immédiat organique. le philothion. Bull. Soc. Hist Nat. Toulouse. 1888:173–180.
-
- Fruton J.S. Contrasts in Scientific Style. Emil Fischer and Franz Hofmeister: Their Research Groups and Their Theory of Protein Structure. Proc. Am. Philos. Soc. JSTOR. 1985;129:313–370. - PubMed
-
- Hunter G., Eagles B.A. Glutathione. A critical Study. J. Biol. Chem. 1927;72:147–166. doi: 10.1016/S0021-9258(18)84368-X. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

