Melatonin Alleviates the Impairment of Muscle Bioenergetics and Protein Quality Control Systems in Leptin-Deficiency-Induced Obesity
- PMID: 38001815
- PMCID: PMC10669624
- DOI: 10.3390/antiox12111962
Melatonin Alleviates the Impairment of Muscle Bioenergetics and Protein Quality Control Systems in Leptin-Deficiency-Induced Obesity
Abstract
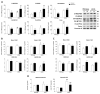
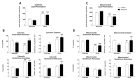
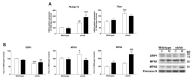
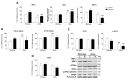
Leptin is critically compromised in the major common forms of obesity. Skeletal muscle is the main effector tissue for energy modification that occurs as a result of the effect of endocrine axes, such as leptin signaling. Our study was carried out using skeletal muscle from a leptin-deficient animal model, in order to ascertain the importance of this hormone and to identify the major skeletal muscle mechanisms affected. We also examined the therapeutic role of melatonin against leptin-induced muscle wasting. Here, we report that leptin deficiency stimulates fatty acid β-oxidation, which results in mitochondrial uncoupling and the suppression of mitochondrial oxidative damage; however, it increases cytosolic oxidative damage. Thus, different nutrient-sensing pathways are disrupted, impairing proteostasis and promoting lipid anabolism, which induces myofiber degeneration and drives oxidative type I fiber conversion. Melatonin treatment plays a significant role in reducing cellular oxidative damage and regulating energy homeostasis and fuel utilization. Melatonin is able to improve both glucose and mitochondrial metabolism and partially restore proteostasis. Taken together, our study demonstrates melatonin to be a decisive mitochondrial function-fate regulator in skeletal muscle, with implications for resembling physiological energy requirements and targeting glycolytic type II fiber recovery.
Keywords: leptin; melatonin; metabolism; mitochondria; obesity; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Melatonin Alleviates Liver Mitochondrial Dysfunction in Leptin-Deficient Mice.Int J Mol Sci. 2024 Aug 8;25(16):8677. doi: 10.3390/ijms25168677. Int J Mol Sci. 2024. PMID: 39201365 Free PMC article.
-
Melatonin Improves Skeletal Muscle Structure and Oxidative Phenotype by Regulating Mitochondrial Dynamics and Autophagy in Zücker Diabetic Fatty Rat.Antioxidants (Basel). 2023 Jul 27;12(8):1499. doi: 10.3390/antiox12081499. Antioxidants (Basel). 2023. PMID: 37627494 Free PMC article.
-
Tbc1d1 deletion suppresses obesity in leptin-deficient mice.Int J Obes (Lond). 2016 Aug;40(8):1242-9. doi: 10.1038/ijo.2016.45. Epub 2016 Mar 22. Int J Obes (Lond). 2016. PMID: 27089993
-
Protective Effects of Melatonin against Obesity-Induced by Leptin Resistance.Behav Brain Res. 2022 Jan 24;417:113598. doi: 10.1016/j.bbr.2021.113598. Epub 2021 Sep 23. Behav Brain Res. 2022. PMID: 34563600 Review.
-
The response of skeletal muscle to leptin.Front Biosci. 2001 Jan 1;6:D90-7. doi: 10.2741/ceddia. Front Biosci. 2001. PMID: 11145919 Review.
Cited by
-
Irisin Protects Musculoskeletal Homeostasis via a Mitochondrial Quality Control Mechanism.Int J Mol Sci. 2024 Sep 20;25(18):10116. doi: 10.3390/ijms251810116. Int J Mol Sci. 2024. PMID: 39337601 Free PMC article. Review.
-
Exerkines and Sarcopenia: Unveiling the Mechanism Behind Exercise-Induced Mitochondrial Homeostasis.Metabolites. 2025 Jan 16;15(1):59. doi: 10.3390/metabo15010059. Metabolites. 2025. PMID: 39852400 Free PMC article. Review.
-
Melatonin Alleviates Liver Mitochondrial Dysfunction in Leptin-Deficient Mice.Int J Mol Sci. 2024 Aug 8;25(16):8677. doi: 10.3390/ijms25168677. Int J Mol Sci. 2024. PMID: 39201365 Free PMC article.
References
-
- Tataranni P.A., Ravussin E. Variability in metabolic rate: Biological sites of regulation. Int. J. Obes. Relat. Metab. Disord. 1995;19((Suppl. S4)):S102–S106. - PubMed
LinkOut - more resources
Full Text Sources

