Differential Modulation of Markers of Oxidative Stress and DNA Damage in Arterial Hypertension
- PMID: 38001818
- PMCID: PMC10669810
- DOI: 10.3390/antiox12111965
Differential Modulation of Markers of Oxidative Stress and DNA Damage in Arterial Hypertension
Abstract
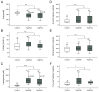
Patients with arterial hypertension have an increased risk of developing tumors, particularly renal cell carcinoma. Arterial hypertension is linked to DNA damage via the generation of oxidative stress, in which an upregulated renin-angiotensin-aldosterone system plays a crucial role. The current study investigated surrogates of oxidative stress and DNA damage in a group of hypertensive patients (HypAll, n = 64) and subgroups of well (HypWell, n = 36) and poorly (HypPoor, n = 28) controlled hypertensive patients compared to healthy controls (n = 8). In addition, a longitudinal analysis was performed with some of the hypertensive patients. Markers for oxidative stress in plasma (SHp, D-ROM, and 3-nitrotyrosine) and urine (8-oxodG, 15-F2t-isoprostane, and malondialdehyde) and markers for DNA damage in lymphocytes (γ-H2AX and micronuclei) were measured. In HypAll, all markers of oxidative stress except malondialdehyde were increased compared to the controls. After adjustment for age, this association was maintained for the protein stress markers SHp and 3-nitrotyrosine. With regard to the markers for DNA damage, there was no difference between HypAll and the controls. Further, no significant differences became apparent in the levels of both oxidative stress and DNA damage between HypWell and HypPoor. Finally, a positive correlation between the development of blood pressure and oxidative stress was observed in the longitudinal study based on the changes in D-ROM and systolic blood pressure. In conclusion, we found increased oxidative stress in extensively treated hypertensive patients correlating with the level of blood-pressure control but no association with DNA damage.
Keywords: 3-nitrotyrosine; 8-oxodG; SHp; high blood pressure; γ-H2AX.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- WHO Global Report on Hypertension: The Race against a Silent Killer. 2023. [(accessed on 29 September 2023)]. Available online: https://www.who.int/publications/i/item/9789240081062.
-
- Weikert S., Boeing H., Pischon T., Weikert C., Olsen A., Tjonneland A., Overvad K., Becker N., Linseisen J., Trichopoulou A., et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am. J. Epidemiol. 2008;167:438–446. doi: 10.1093/aje/kwm321. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

