Ferrous Ion Alleviates Lipid Deposition and Inflammatory Responses Caused by a High Cottonseed Meal Diet by Modulating Hepatic Iron Transport Homeostasis and Controlling Ferroptosis in Juvenile Ctenopharyngodon idellus
- PMID: 38001821
- PMCID: PMC10669718
- DOI: 10.3390/antiox12111968
Ferrous Ion Alleviates Lipid Deposition and Inflammatory Responses Caused by a High Cottonseed Meal Diet by Modulating Hepatic Iron Transport Homeostasis and Controlling Ferroptosis in Juvenile Ctenopharyngodon idellus
Abstract
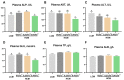
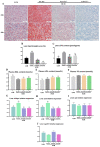
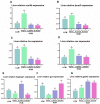
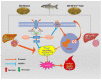
To investigate the mechanisms through which ferrous ion (Fe2+) addition improves the utilization of a cottonseed meal (CSM) diet, two experimental diets with equal nitrogen and energy content (low-cottonseed meal (LCM) and high-cottonseed meal (HCM) diets, respectively) containing 16.31% and 38.46% CSM were prepared. Additionally, the HCM diet was supplemented with graded levels of FeSO4·7H2O to establish two different Fe2+ supplementation groups (HCM + 0.2%Fe2+ and HCM + 0.4%Fe2+). Juvenile Ctenopharyngodon idellus (grass carps) (5.0 ± 0.5 g) were fed one of these four diets (HCM, LCM, HCM + 0.2%Fe2+ and HCM + 0.4%Fe2+ diets) for eight weeks. Our findings revealed that the HCM diet significantly increased lipid peroxide (LPO) concentration and the expression of lipogenic genes, e.g., sterol regulatory element binding transcription factor 1 (srebp1) and stearoyl-CoA desaturase (scd), leading to excessive lipid droplet deposition in the liver (p < 0.05). However, these effects were significantly reduced in the HCM + 0.2%Fe2+ and HCM + 0.4%Fe2+ groups (p < 0.05). Plasma high-density lipoprotein (HDL) concentration was also significantly lower in the HCM and HCM + 0.2%Fe2+ groups compared to the LCM group (p < 0.05), whereas low-density lipoprotein (LDL) concentration was significantly higher in the HCM + 0.2%Fe2+ and HCM + 0.4%Fe2+ groups than in the LCM group (p < 0.05). Furthermore, the plasma levels of liver functional indices, including alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and glucose (GLU), were significantly lower in the HCM + 0.4%Fe2+ group (p < 0.05). Regarding the expression of genes related to iron transport regulation, transferrin 2 (tfr2) expression in the HCM group and Fe2+ supplementation groups were significantly suppressed compared to the LCM group (p < 0.05). The addition of 0.4% Fe2+ in the HCM diet activated hepcidin expression and suppressed ferroportin-1 (fpn1) expression (p < 0.05). Compared to the LCM group, the expression of genes associated with ferroptosis and inflammation, including acyl-CoA synthetase long-chain family member 4b (acsl4b), lysophosphatidylcholine acyltransferase 3 (lpcat3), cyclooxygenase (cox), interleukin 1β (il-1β), and nuclear factor kappa b (nfκb), were significantly increased in the HCM group (p < 0.05), whereas Fe2+ supplementation in the HCM diet significantly inhibited their expression (p < 0.05) and significantly suppressed lipoxygenase (lox) expression (p < 0.05). Compared with the HCM group without Fe2+ supplementation, Fe2+ supplementation in the HCM diet significantly upregulated the expression of genes associated with ferroptosis, such as heat shock protein beta-associated protein1 (hspbap1), glutamate cysteine ligase (gcl), and glutathione peroxidase 4a (gpx4a) (p < 0.05), and significantly decreased the expression of the inflammation-related genes interleukin 15/10 (il-15/il-10) (p < 0.05). In conclusion, FeSO4·7H2O supplementation in the HCM diet maintained iron transport and homeostasis in the liver of juvenile grass carps, thus reducing the occurrence of ferroptosis and alleviating hepatic lipid deposition and inflammatory responses caused by high dietary CSM contents.
Keywords: herbivorous fish; inflammation; ion transporter; lipid metabolism; plant protein source.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effects of dietary lysolecithin on growth performance, serum biochemical indexes, antioxidant capacity, lipid metabolism and inflammation-related genes expression of juvenile large yellow croaker (Larimichthys crocea).Fish Shellfish Immunol. 2022 Sep;128:50-59. doi: 10.1016/j.fsi.2022.07.020. Epub 2022 Jul 14. Fish Shellfish Immunol. 2022. PMID: 35843522
-
Dietary ferulic acid supplementation improved cottonseed meal-based diet utilization by enhancing intestinal physical barrier function and liver antioxidant capacity in grass carp (Ctenopharyngodon Idellus).Front Physiol. 2022 Aug 22;13:922037. doi: 10.3389/fphys.2022.922037. eCollection 2022. Front Physiol. 2022. PMID: 36072855 Free PMC article.
-
Impact of exogenous lipase supplementation on growth, intestinal function, mucosal immune and physical barrier, and related signaling molecules mRNA expression of young grass carp (Ctenopharyngodon idella).Fish Shellfish Immunol. 2016 Aug;55:88-105. doi: 10.1016/j.fsi.2016.05.006. Epub 2016 May 7. Fish Shellfish Immunol. 2016. PMID: 27164217
-
Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-κB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila.Fish Shellfish Immunol. 2020 Feb;97:540-553. doi: 10.1016/j.fsi.2019.12.074. Epub 2019 Dec 24. Fish Shellfish Immunol. 2020. PMID: 31881329
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
Cited by
-
Use of Cottonseed Meal in Feeding Yellow-Feathered Broilers: Effects on Performance Parameters, Digestibility and Meat Quality.Vet Sci. 2025 Apr 27;12(5):416. doi: 10.3390/vetsci12050416. Vet Sci. 2025. PMID: 40431509 Free PMC article.
References
-
- Dani D. A review on replacing fish meal in aqua feeds using plant protein sources. Int. J. Fish. Aquat. Stud. 2018;6:164–179.
-
- Kumar B.P., Ramudu K.R., Devi B.C. Mini review on incorporation of cotton seed meal, an alternative to fish meal in aquaculture feeds. Int. J. Biol. Res. 2014;22:99–105.
-
- Dawood M.A.O., Koshio S. Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquacult. 2020;122:987–1002. doi: 10.1111/raq.12368. - DOI
-
- Lim S.J., Lee K.J. Partial replacement of fish meal by cottonseed meal and soybean meal with iron and phytase supplementation for parrot fish Oplegnathus fasciatus. Aquaculture. 2009;290:283–289. doi: 10.1016/j.aquaculture.2009.02.018. - DOI
-
- Hardy R.W. Worldwide fish meal production outlook and the use of alternative protein meals for aquaculture. Av. Nutr. Acuicola. 2006:410–419.
Grants and funding
LinkOut - more resources
Full Text Sources

