Azadirachtin Attenuates Carcinogen Benzo(a) Pyrene-Induced DNA Damage, Cell Cycle Arrest, Apoptosis, Inflammatory, Metabolic, and Oxidative Stress in HepG2 Cells
- PMID: 38001854
- PMCID: PMC10669168
- DOI: 10.3390/antiox12112001
Azadirachtin Attenuates Carcinogen Benzo(a) Pyrene-Induced DNA Damage, Cell Cycle Arrest, Apoptosis, Inflammatory, Metabolic, and Oxidative Stress in HepG2 Cells
Abstract
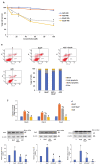
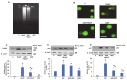
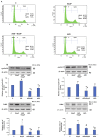
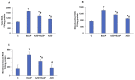
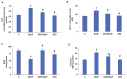
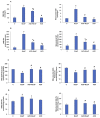
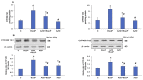
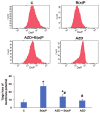
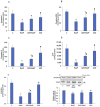
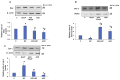
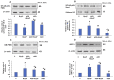
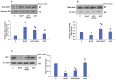
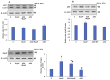
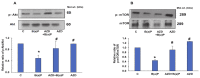
Azadirachtin (AZD), a limonoid from the versatile, tropical neem tree (Azadirachta indica), is well known for its many medicinal, and pharmacological effects. Its effects as an anti-oxidant, anti-inflammatory, and anti-cancer agent are well known. However, not many studies have explored the effects of AZD on toxicities induced by benzo(a)pyrene (B(a)P), a toxic component of cigarette smoke known to cause DNA damage and cell cycle arrest, leading to different kinds of cancer. In the present study, using HepG2 cells, we investigated the protective effects of Azadirachtin (AZD) against B(a)P-induced oxidative/nitrosative and metabolic stress and mitochondrial dysfunction. Treatment with 25 µM B(a)P for 24 h demonstrated an increased production of reactive oxygen species (ROS), followed by increased lipid peroxidation and DNA damage presumably, due to the increased metabolic activation of B(a)P by CYP 450 1A1/1A2 enzymes. We also observed intrinsic and extrinsic apoptosis, alterations in glutathione-dependent redox homeostasis, cell cycle arrest, and inflammation after B(a)P treatment. Cells treated with 25 µM AZD for 24 h showed decreased oxidative stress and apoptosis, partial protection from DNA damage, and an improvement in mitochondrial functions and bioenergetics. The improvement in antioxidant status, anti-inflammatory potential, and alterations in cell cycle regulatory markers qualify AZD as a potential therapeutic in combination with anti-cancer drugs.
Keywords: Azadirachtin; DNA damage; HepG2 cells; apoptosis; benzo(a)pyrene; mitochondrial dysfunction; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Azadirachtin Attenuates Lipopolysaccharide-Induced ROS Production, DNA Damage, and Apoptosis by Regulating JNK/Akt and AMPK/mTOR-Dependent Pathways in Rin-5F Pancreatic Beta Cells.Biomedicines. 2021 Dec 18;9(12):1943. doi: 10.3390/biomedicines9121943. Biomedicines. 2021. PMID: 34944759 Free PMC article.
-
Alterations in Inflammatory Cytokines and Redox Homeostasis in LPS-Induced Pancreatic Beta-Cell Toxicity and Mitochondrial Stress: Protection by Azadirachtin.Front Cell Dev Biol. 2022 Jun 20;10:867608. doi: 10.3389/fcell.2022.867608. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35794865 Free PMC article.
-
Effects of taurine against benzo[α]pyrene-induced cell cycle arrest and reactive oxygen species-mediated nuclear factor-kappa B apoptosis via reduction of mitochondrial stress in A549 cells.Chin J Physiol. 2022 Jul-Aug;65(4):199-208. doi: 10.4103/0304-4920.354803. Chin J Physiol. 2022. PMID: 36073568
-
Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]pyrene-Induced Oxidative Stress.Molecules. 2022 Feb 18;27(4):1379. doi: 10.3390/molecules27041379. Molecules. 2022. PMID: 35209168 Free PMC article. Review.
-
Free radical biology in neurological manifestations: mechanisms to therapeutics interventions.Environ Sci Pollut Res Int. 2022 Sep;29(41):62160-62207. doi: 10.1007/s11356-021-16693-2. Epub 2021 Oct 6. Environ Sci Pollut Res Int. 2022. PMID: 34617231 Review.
Cited by
-
Sucralose Promotes Benzo(a)Pyrene-Induced Renal Toxicity in Mice by Regulating P-glycoprotein.Antioxidants (Basel). 2025 Apr 16;14(4):474. doi: 10.3390/antiox14040474. Antioxidants (Basel). 2025. PMID: 40298799 Free PMC article.
-
Common immunological and prognostic features of lung and bladder cancer via smoking-related genes: PRR11 gene as potential immunotherapeutic target.J Cell Mol Med. 2024 May;28(10):e18384. doi: 10.1111/jcmm.18384. J Cell Mol Med. 2024. PMID: 38760964 Free PMC article.
References
-
- Chhikara B.S., Parang K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2023;10:451
-
- U.S. EPA . IRIS Toxicological Review of Benzo[A]Pyrene (Final Report) U.S. Environmental Protection Agency; Washington, DC, USA: 2017. EPA/635/R-17/003F.
-
- Liu B.-Y., Chiou J.-Z., Huang K.-M., Chen T.-Y., Hwang D.-F. Effects of Taurine against Benzo[α]Pyrene-Induced Cell Cycle Arrest and Reactive Oxygen Species-Mediated Nuclear Factor-Kappa B Apoptosis via Reduction of Mitochondrial Stress in A549 Cells. Chin. J. Physiol. 2022;65:199. doi: 10.4103/0304-4920.354803. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

