Exploring the Use of Hydroxytyrosol and Some of Its Esters in Food-Grade Nanoemulsions: Establishing Connection between Structure and Efficiency
- PMID: 38001855
- PMCID: PMC10669426
- DOI: 10.3390/antiox12112002
Exploring the Use of Hydroxytyrosol and Some of Its Esters in Food-Grade Nanoemulsions: Establishing Connection between Structure and Efficiency
Abstract
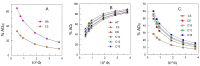
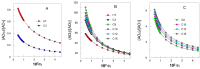
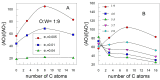
The efficiency of HT and that of some of its hydrophobic derivatives and their distribution and effective concentrations were investigated in fish oil-in-water nanoemulsions. For this purpose, we carried out two sets of independent, but complementary, kinetic experiments in the same intact fish nanoemulsions. In one of them, we monitored the progress of lipid oxidation in intact nanoemulsions by monitoring the formation of conjugated dienes with time. In the second set of experiments, we determined the distributions and effective concentrations of HT and its derivatives in the same intact nanoemulsions as those employed in the oxidation experiments. Results show that the antioxidant efficiency is consistent with the "cut-off" effect-the efficiency of HT derivatives increases upon increasing their hydrophobicity up to the octyl derivative after which a further increase in the hydrophobicity decreases their efficiency. Results indicate that the effective interfacial concentration is the main factor controlling the efficiency of the antioxidants and that such efficiency strongly depends on the surfactant concentration and on the oil-to-water (o/w) ratio employed to prepare the nanoemulsions.
Keywords: antioxidant efficiency; food-grade nanoemulsions; hydroxytyrosol; lipid oxidation; partition constants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


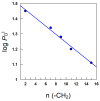
 0.047,
0.047,  0.094,
0.094,  0.141,
0.141,  0.188,
0.188,  0.235,
0.235,  0.282, T = 25 °C. Solid lines were drawn to aid the eye. (B) Variation in the percentage of DPPH● as a function of the AO/DPPH● ratio at different reaction times and the corresponding linear fits (solid lines).
0.282, T = 25 °C. Solid lines were drawn to aid the eye. (B) Variation in the percentage of DPPH● as a function of the AO/DPPH● ratio at different reaction times and the corresponding linear fits (solid lines).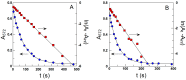
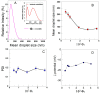

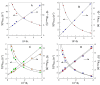

Similar articles
-
Interfacial Concentrations of Hydroxytyrosol Derivatives in Fish Oil-in-Water Emulsions and Nanoemulsions and Its Influence on Their Lipid Oxidation: Droplet Size Effects.Foods. 2020 Dec 18;9(12):1897. doi: 10.3390/foods9121897. Foods. 2020. PMID: 33353202 Free PMC article.
-
Unexpected Antioxidant Efficiency of Chlorogenic Acid Phenolipids in Fish Oil-in-Water Nanoemulsions: An Example of How Relatively Low Interfacial Concentrations Can Make Antioxidants to Be Inefficient.Molecules. 2022 Jan 27;27(3):861. doi: 10.3390/molecules27030861. Molecules. 2022. PMID: 35164119 Free PMC article.
-
Enhancement of the antioxidant efficiency of gallic acid derivatives in intact fish oil-in-water emulsions through optimization of their interfacial concentrations.Food Funct. 2018 Aug 15;9(8):4429-4442. doi: 10.1039/c8fo00977e. Food Funct. 2018. PMID: 30070303
-
Interfacial Concentrations of Hydroxytyrosol and Its Lipophilic Esters in Intact Olive Oil-in-Water Emulsions: Effects of Antioxidant Hydrophobicity, Surfactant Concentration, and the Oil-to-Water Ratio on the Oxidative Stability of the Emulsions.J Agric Food Chem. 2016 Jun 29;64(25):5274-83. doi: 10.1021/acs.jafc.6b01468. Epub 2016 Jun 15. J Agric Food Chem. 2016. PMID: 27157893
-
Advances in the control of lipid peroxidation in oil-in-water emulsions: kinetic approaches†.Crit Rev Food Sci Nutr. 2023;63(23):6252-6284. doi: 10.1080/10408398.2022.2029827. Epub 2022 Feb 1. Crit Rev Food Sci Nutr. 2023. PMID: 35104177 Review.
Cited by
-
Design, Synthesis, and Biological Evaluation of Novel Hydroxytyrosol Derivatives as Protectors for Vascular Endothelium Against Lipid Overload.Drug Des Devel Ther. 2025 Apr 1;19:2433-2452. doi: 10.2147/DDDT.S500670. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40190807 Free PMC article.
References
-
- Sarker S.D., Nahar L. Medicinal Natural Products: A Disease-Focused Approach. Elsevier Science; Amsterdam, The Netherlands: 2020.
-
- Brahmachari G. Discovery and Development of Anti-Inflammatory Agents from Natural Products. Elsevier Science; Amsterdam, The Netherlands: 2019.
-
- Gopi S., Balakrishnan P. Handbook of Nutraceuticals and Natural Products. Wiley; Hoboken, NJ, USA: 2022.
-
- Schwab W., Lange B.M., Wüst M. Biotechnology of Natural Products. Springer International Publishing; Berlin/Heidelberg, Germany: 2017.
-
- Banerjee R., Verma A.K., Siddiqui M.W. Natural Antioxidants: Applications in Foods of Animal Origin. Apple Academic Press; Cambridge, MA, USA: 2017.
Grants and funding
LinkOut - more resources
Full Text Sources

