Depletion of Labile Iron Induces Replication Stress and Enhances Responses to Chemoradiation in Non-Small-Cell Lung Cancer
- PMID: 38001858
- PMCID: PMC10669787
- DOI: 10.3390/antiox12112005
Depletion of Labile Iron Induces Replication Stress and Enhances Responses to Chemoradiation in Non-Small-Cell Lung Cancer
Abstract
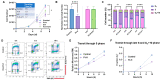
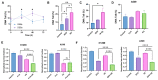
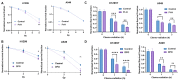
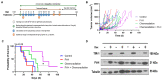
The intracellular redox-active labile iron pool (LIP) is weakly chelated and available for integration into the iron metalloproteins that are involved in diverse cellular processes, including cancer cell-specific metabolic oxidative stress. Abnormal iron metabolism and elevated LIP levels are linked to the poor survival of lung cancer patients, yet the underlying mechanisms remain unclear. Depletion of the LIP in non-small-cell lung cancer cell lines using the doxycycline-inducible overexpression of the ferritin heavy chain (Ft-H) (H1299 and H292), or treatment with deferoxamine (DFO) (H1299 and A549), inhibited cell growth and decreased clonogenic survival. The Ft-H overexpression-induced inhibition of H1299 and H292 cell growth was also accompanied by a significant delay in transit through the S-phase. In addition, both Ft-H overexpression and DFO in H1299 resulted in increased single- and double-strand DNA breaks, supporting the involvement of replication stress in the response to LIP depletion. The Ft-H and DFO treatment also sensitized H1299 to VE-821, an inhibitor of ataxia telangiectasis and Rad2-related (ATR) kinase, highlighting the potential of LIP depletion, combined with DNA damage response modifiers, to alter lung cancer cell responses. In contrast, only DFO treatment effectively reduced the LIP, clonogenic survival, cell growth, and sensitivity to VE-821 in A549 non-small-cell lung cancer cells. Importantly, the Ft-H and DFO sensitized both H1299 and A549 to chemoradiation in vitro, and Ft-H overexpression increased the efficacy of chemoradiation in vivo in H1299. These results support the hypothesis that the depletion of the LIP can induce genomic instability, cell death, and potentiate therapeutic responses to chemoradiation in NSCLC.
Keywords: DNA damage; cell cycle; chemoradiation; deferoxamine; ferritin heavy chain; holo-transferrin; iron chelator; labile iron pool; lung cancer; replication stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schoenfeld J.D., Sibenaller Z.A., Mapuskar K.A., Wagner B.A., Cramer-Morales K.L., Furqan M., Sandhu S., Carlisle T.L., Smith M.C., Abu Hejleh T., et al. O2− and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell. 2017;31:487–500.e488. doi: 10.1016/j.ccell.2017.02.018. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

