c-Abl Phosphorylates MFN2 to Regulate Mitochondrial Morphology in Cells under Endoplasmic Reticulum and Oxidative Stress, Impacting Cell Survival and Neurodegeneration
- PMID: 38001860
- PMCID: PMC10669615
- DOI: 10.3390/antiox12112007
c-Abl Phosphorylates MFN2 to Regulate Mitochondrial Morphology in Cells under Endoplasmic Reticulum and Oxidative Stress, Impacting Cell Survival and Neurodegeneration
Abstract
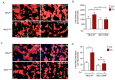
The endoplasmic reticulum is a subcellular organelle key in the control of synthesis, folding, and sorting of proteins. Under endoplasmic reticulum stress, an adaptative unfolded protein response is activated; however, if this activation is prolonged, cells can undergo cell death, in part due to oxidative stress and mitochondrial fragmentation. Here, we report that endoplasmic reticulum stress activates c-Abl tyrosine kinase, inducing its translocation to mitochondria. We found that endoplasmic reticulum stress-activated c-Abl interacts with and phosphorylates the mitochondrial fusion protein MFN2, resulting in mitochondrial fragmentation and apoptosis. Moreover, the pharmacological or genetic inhibition of c-Abl prevents MFN2 phosphorylation, mitochondrial fragmentation, and apoptosis in cells under endoplasmic reticulum stress. Finally, in the amyotrophic lateral sclerosis mouse model, where endoplasmic reticulum and oxidative stress has been linked to neuronal cell death, we demonstrated that the administration of c-Abl inhibitor neurotinib delays the onset of symptoms. Our results uncovered a function of c-Abl in the crosstalk between endoplasmic reticulum stress and mitochondrial dynamics via MFN2 phosphorylation.
Keywords: amyotrophic lateral sclerosis; apoptosis; c-Abl; endoplasmic reticulum stress; mitochondrial fusion; mitofusin 2.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Neurotinib is under the patent WO2019/173761 A1. D.R.G. consults and/or has equity in Ventus Pharmaceuticals and Sonata Therapeutics, and receives support from Horizon Pharmaceuticals.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

