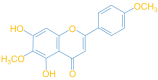
Pectolinarigenin Improves Oxidative Stress and Apoptosis in Mouse NSC-34 Motor Neuron Cell Lines Induced by C9-ALS-Associated Proline-Arginine Dipeptide Repeat Proteins by Enhancing Mitochondrial Fusion Mediated via the SIRT3/OPA1 Axis
- PMID: 38001861
- PMCID: PMC10669908
- DOI: 10.3390/antiox12112008
Pectolinarigenin Improves Oxidative Stress and Apoptosis in Mouse NSC-34 Motor Neuron Cell Lines Induced by C9-ALS-Associated Proline-Arginine Dipeptide Repeat Proteins by Enhancing Mitochondrial Fusion Mediated via the SIRT3/OPA1 Axis
Abstract
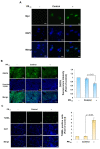
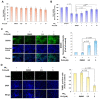
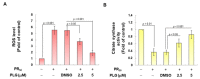
Amyotrophic lateral sclerosis (ALS) is considered a fatal progressive degeneration of motor neurons (MN) caused by oxidative stress and mitochondrial dysfunction. There are currently no treatments available. The most common inherited form of ALS is the C9orf72 mutation (C9-ALS). The proline-arginine dipeptide repeat protein (PR-DPR) produced by C9-ALS has been confirmed to be a functionally acquired pathogenic factor that can cause increased ROS, mitochondrial defects, and apoptosis in motor neurons. Pectolinarigenin (PLG) from the traditional medicinal herb Linaria vulgaris has antioxidant and anti-apoptotic properties. I established a mouse NSC-34 motor neuron cell line model expressing PR-DPR and confirmed the neuroprotective effect of PLG. The results showed that ROS production and apoptosis caused by PR-DPR could be improved by PLG treatment. In terms of mechanism research, PR-DPR inhibited the activity of the mitochondrial fusion proteins OPA1 and mitofusin 2. Conversely, the expression of fission protein fission 1 and dynamin-related protein 1 (DRP1) increased. However, PLG treatment reversed these effects. Furthermore, I found that PLG increased the expression and deacetylation of OPA1. Deacetylation of OPA1 enhances mitochondrial fusion and resistance to apoptosis. Finally, transfection with Sirt3 small interfering RNA abolished the neuroprotective effects of PLG. In summary, the mechanism by which PLG alleviates PR-DPR toxicity is mainly achieved by activating the SIRT3/OPA1 axis to regulate the balance of mitochondrial dynamics. Taken together, the potential of PLG in preclinical studies for C9-ALS drug development deserves further evaluation.
Keywords: C9orf72; OPA1; ROS; SIRT3; acetylation; amyotrophic lateral sclerosis (ALS); apoptosis; mitochondrial dynamics; pectolinarigenin; proline–arginine dipeptide repeat protein (PR-DPR).
Conflict of interest statement
The author declares no conflict of interest.
Figures





Similar articles
-
C9-ALS-Associated Proline-Arginine Dipeptide Repeat Protein Induces Activation of NLRP3 Inflammasome of HMC3 Microglia Cells by Binding of Complement Component 1 Q Subcomponent-Binding Protein (C1QBP), and Syringin Prevents This Effect.Cells. 2022 Oct 5;11(19):3128. doi: 10.3390/cells11193128. Cells. 2022. PMID: 36231090 Free PMC article.
-
Interaction of the C9orf72-Amyotrophic Lateral Sclerosis-Related Proline-Arginine Dipeptide Repeat Protein with the RNA-Binding Protein NOVA1 Causes Decreased Expression of UNC13A Due to Enhanced Inclusion of Cryptic Exons, Which Is Reversed by Betulin Treatment.Cells. 2023 Oct 18;12(20):2476. doi: 10.3390/cells12202476. Cells. 2023. PMID: 37887320 Free PMC article.
-
C9orf72 Dipeptide Repeats Cause Selective Neurodegeneration and Cell-Autonomous Excitotoxicity in Drosophila Glutamatergic Neurons.J Neurosci. 2018 Aug 29;38(35):7741-7752. doi: 10.1523/JNEUROSCI.0908-18.2018. Epub 2018 Jul 23. J Neurosci. 2018. PMID: 30037833 Free PMC article.
-
Arginine-rich dipeptide-repeat proteins as phase disruptors in C9-ALS/FTD.Emerg Top Life Sci. 2020 Dec 11;4(3):293-305. doi: 10.1042/ETLS20190167. Emerg Top Life Sci. 2020. PMID: 32639008 Free PMC article. Review.
-
C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels.Autophagy. 2021 Nov;17(11):3306-3322. doi: 10.1080/15548627.2021.1872189. Epub 2021 Feb 26. Autophagy. 2021. PMID: 33632058 Free PMC article. Review.
Cited by
-
Cardiac-specific PFKFB3 overexpression prevents diabetic cardiomyopathy via enhancing OPA1 stabilization mediated by K6-linked ubiquitination.Cell Mol Life Sci. 2024 May 22;81(1):228. doi: 10.1007/s00018-024-05257-5. Cell Mol Life Sci. 2024. PMID: 38777955 Free PMC article.
-
Mitochondrial DNA leakage: underlying mechanisms and therapeutic implications in neurological disorders.J Neuroinflammation. 2025 Feb 7;22(1):34. doi: 10.1186/s12974-025-03363-0. J Neuroinflammation. 2025. PMID: 39920753 Free PMC article. Review.
-
Mechanism of USP11 regulated SIRT3/ROS in drug resistance of acute myeloid leukemia.Clin Exp Med. 2025 Jul 30;25(1):267. doi: 10.1007/s10238-025-01814-9. Clin Exp Med. 2025. PMID: 40736712 Free PMC article.
References
-
- Fu R.H., Tsai C.W., Chiu S.C., Liu S.P., Chiang Y.T., Kuo Y.H., Shyu W.C., Lin S.Z. C9-ALS-Associated Proline-Arginine Dipeptide Repeat Protein Induces Activation of NLRP3 Inflammasome of HMC3 Microglia Cells by Binding of Complement Component 1 Q Subcomponent-Binding Protein (C1QBP), and Syringin Prevents This Effect. Cells. 2022;11:3128. doi: 10.3390/cells11193128. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

