Antioxidants of Non-Enzymatic Nature: Their Function in Higher Plant Cells and the Ways of Boosting Their Biosynthesis
- PMID: 38001867
- PMCID: PMC10669185
- DOI: 10.3390/antiox12112014
Antioxidants of Non-Enzymatic Nature: Their Function in Higher Plant Cells and the Ways of Boosting Their Biosynthesis
Abstract
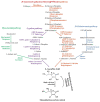
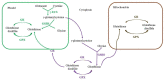
Plants are exposed to a variety of abiotic and biotic stresses leading to increased formation of reactive oxygen species (ROS) in plant cells. ROS are capable of oxidizing proteins, pigments, lipids, nucleic acids, and other cell molecules, disrupting their functional activity. During the process of evolution, numerous antioxidant systems were formed in plants, including antioxidant enzymes and low molecular weight non-enzymatic antioxidants. Antioxidant systems perform neutralization of ROS and therefore prevent oxidative damage of cell components. In the present review, we focus on the biosynthesis of non-enzymatic antioxidants in higher plants cells such as ascorbic acid (vitamin C), glutathione, flavonoids, isoprenoids, carotenoids, tocopherol (vitamin E), ubiquinone, and plastoquinone. Their functioning and their reactivity with respect to individual ROS will be described. This review is also devoted to the modern genetic engineering methods, which are widely used to change the quantitative and qualitative content of the non-enzymatic antioxidants in cultivated plants. These methods allow various plant lines with given properties to be obtained in a rather short time. The most successful approaches for plant transgenesis and plant genome editing for the enhancement of biosynthesis and the content of these antioxidants are discussed.
Keywords: CRISPR/Cas9; antioxidants; ascorbic acid; carotenoids; flavonoids; glutathione; higher plants; isoprenoids; plastoquinone; reactive oxygen species; tocopherol; transgenesis; ubiquinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Oxidative damage and antioxidative system in algae.Toxicol Rep. 2019 Oct 24;6:1309-1313. doi: 10.1016/j.toxrep.2019.10.001. eCollection 2019. Toxicol Rep. 2019. PMID: 31993331 Free PMC article. Review.
-
The ascorbate-glutathione-α-tocopherol triad in abiotic stress response.Int J Mol Sci. 2012;13(4):4458-4483. doi: 10.3390/ijms13044458. Epub 2012 Apr 10. Int J Mol Sci. 2012. PMID: 22605990 Free PMC article. Review.
-
Antioxidant Defenses in Plants with Attention to Prunus and Citrus spp.Antioxidants (Basel). 2013 Nov 26;2(4):340-69. doi: 10.3390/antiox2040340. Antioxidants (Basel). 2013. PMID: 26784469 Free PMC article. Review.
-
Antioxidants, oxidative damage and oxygen deprivation stress: a review.Ann Bot. 2003 Jan;91 Spec No(2):179-94. doi: 10.1093/aob/mcf118. Ann Bot. 2003. PMID: 12509339 Free PMC article. Review.
-
Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective.Biology (Basel). 2022 Jan 18;11(2):155. doi: 10.3390/biology11020155. Biology (Basel). 2022. PMID: 35205022 Free PMC article. Review.
Cited by
-
Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective.Life (Basel). 2025 Jan 16;15(1):113. doi: 10.3390/life15010113. Life (Basel). 2025. PMID: 39860053 Free PMC article. Review.
-
Impact of plant-derived antioxidants on heart aging: a mechanistic outlook.Front Pharmacol. 2025 Mar 21;16:1524584. doi: 10.3389/fphar.2025.1524584. eCollection 2025. Front Pharmacol. 2025. PMID: 40191425 Free PMC article. Review.
-
Silicon application improves tomato yield and nutritional quality.BMC Plant Biol. 2025 Feb 25;25(1):252. doi: 10.1186/s12870-025-06249-8. BMC Plant Biol. 2025. PMID: 39994511 Free PMC article.
-
A Review on Bioactive Anthraquinone and Derivatives as the Regulators for ROS.Molecules. 2023 Dec 17;28(24):8139. doi: 10.3390/molecules28248139. Molecules. 2023. PMID: 38138627 Free PMC article. Review.
-
RIP5 Interacts with REL1 and Negatively Regulates Drought Tolerance in Rice.Cells. 2024 May 21;13(11):887. doi: 10.3390/cells13110887. Cells. 2024. PMID: 38891020 Free PMC article.
References
-
- Borisova M.M.M., Kozuleva M.A., Rudenko N.N., Naydov I.A., Klenina I.B., Ivanov B.N. Photosynthetic Electron Flow to Oxygen and Diffusion of Hydrogen Peroxide through the Chloroplast Envelope via Aquaporins. Biochim. Biophys. Acta (BBA)–Bioenerg. 2012;1817:1314–1321. doi: 10.1016/j.bbabio.2012.02.036. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

