The Role and Therapeutic Implications of Inflammation in the Pathogenesis of Brain Arteriovenous Malformations
- PMID: 38001877
- PMCID: PMC10669898
- DOI: 10.3390/biomedicines11112876
The Role and Therapeutic Implications of Inflammation in the Pathogenesis of Brain Arteriovenous Malformations
Abstract
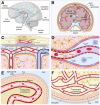
Brain arteriovenous malformations (bAVMs) are focal vascular lesions composed of abnormal vascular channels without an intervening capillary network. As a result, high-pressure arterial blood shunts directly into the venous outflow system. These high-flow, low-resistance shunts are composed of dilated, tortuous, and fragile vessels, which are prone to rupture. BAVMs are a leading cause of hemorrhagic stroke in children and young adults. Current treatments for bAVMs are limited to surgery, embolization, and radiosurgery, although even these options are not viable for ~20% of AVM patients due to excessive risk. Critically, inflammation has been suggested to contribute to lesion progression. Here we summarize the current literature discussing the role of the immune system in bAVM pathogenesis and lesion progression, as well as the potential for targeting inflammation to prevent bAVM rupture and intracranial hemorrhage. We conclude by proposing that a dysfunctional endothelium, which harbors the somatic mutations that have been shown to give rise to sporadic bAVMs, may drive disease development and progression by altering the immune status of the brain.
Keywords: blood brain barrier; brain arteriovenous malformation; cerebrovascular; endothelium; inflammation; microglia; neurovascular unit; vascular.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
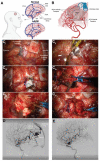
Reductions in brain pericytes are associated with arteriovenous malformation vascular instability.J Neurosurg. 2018 Dec 1;129(6):1464-1474. doi: 10.3171/2017.6.JNS17860. Epub 2018 Jan 5. J Neurosurg. 2018. PMID: 29303444 Free PMC article.
-
Pathogenesis and radiobiology of brain arteriovenous malformations: implications for risk stratification in natural history and posttreatment course.Neurosurg Focus. 2009 May;26(5):E9. doi: 10.3171/2009.2.FOCUS0926. Neurosurg Focus. 2009. PMID: 19409010 Review.
-
Management of intracranial aneurysms associated with arteriovenous malformations.Neurosurg Focus. 2014 Sep;37(3):E11. doi: 10.3171/2014.6.FOCUS14165. Neurosurg Focus. 2014. PMID: 25175430
-
Critical review of brain AVM surgery, surgical results and natural history in 2017.Acta Neurochir (Wien). 2017 Aug;159(8):1457-1478. doi: 10.1007/s00701-017-3217-x. Epub 2017 May 29. Acta Neurochir (Wien). 2017. PMID: 28555270 Review.
-
Current Concepts and Perspectives on Brain Arteriovenous Malformations: A Review of Pathogenesis and Multidisciplinary Treatment.World Neurosurg. 2022 Mar;159:314-326. doi: 10.1016/j.wneu.2021.07.106. Epub 2021 Jul 30. World Neurosurg. 2022. PMID: 34339893 Review.
Cited by
-
Unraveling the transcriptomic landscape of brain vascular cells in dementia: A systematic review.Alzheimers Dement. 2025 Feb;21(2):e14512. doi: 10.1002/alz.14512. Epub 2025 Jan 14. Alzheimers Dement. 2025. PMID: 39807599 Free PMC article.
-
Untangling sporadic brain arteriovenous malformations: towards targeting the KRAS/MAPK pathway.Front Surg. 2024 Dec 2;11:1504612. doi: 10.3389/fsurg.2024.1504612. eCollection 2024. Front Surg. 2024. PMID: 39687326 Free PMC article. Review.
-
Long-Term Outcomes of Stereotactic Radiosurgery Focused Treatment of Brain Arteriovenous Malformations Based on Rupture Status: A Systematic Review and Meta-Analysis.Transl Stroke Res. 2025 Oct;16(5):1666-1688. doi: 10.1007/s12975-025-01339-z. Epub 2025 Mar 20. Transl Stroke Res. 2025. PMID: 40111720
-
Lack of correlation between cerebral arteriovenous malformation angioarchitectural and hemodynamic characteristics and systemic inflammation.Acta Neurochir (Wien). 2025 Apr 15;167(1):106. doi: 10.1007/s00701-025-06464-0. Acta Neurochir (Wien). 2025. PMID: 40229563 Free PMC article.
-
Defining the Role of Oral Pathway Inhibitors as Targeted Therapeutics in Arteriovenous Malformation Care.Biomedicines. 2024 Jun 11;12(6):1289. doi: 10.3390/biomedicines12061289. Biomedicines. 2024. PMID: 38927496 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

