Supraorbital Basosquamous Carcinoma Treated with Cemiplimab Followed by Sonidegib: A Case Report and Review of the Literature
- PMID: 38001904
- PMCID: PMC10669660
- DOI: 10.3390/biomedicines11112903
Supraorbital Basosquamous Carcinoma Treated with Cemiplimab Followed by Sonidegib: A Case Report and Review of the Literature
Abstract
Basal cell carcinoma (BCC) is a skin cancer with low local aggressiveness and a low tendency to metastasize. Basosquamous Carcinoma (BSC) represents an aggressive histological subtype of BCC with intermediate features between Squamous Cell Carcinoma (SCC) and BCC. Cemiplimab is currently approved as first-line therapy in SCC and second-line therapy in BCC patients who have progressed on or are intolerant of a Hedgehog pathway Inhibitor (HHI). Our study describes the case of a 59-year-old man with BSC who was successfully treated with 5 cycles of Cemiplimab as first-line therapy and Sonidegib as second-line therapy. Currently, the efficacy of Cemiplimab against BSC and other histopathological subtypes of BCC has not been fully elucidated, as has the role of sequential or combination therapy with Cemiplimab and HHI in the management of BSC. The aim of this case report is to highlight the need to outline the use of checkpoint inhibitors in BCCs and focus attention on the synergistic role of Cemiplimab and HHIs in such a controversial entity as BSC.
Keywords: Basal cell carcinoma; Cemiplimab; Sonidegib; Squamous Cell Carcinoma; basosquamous carcinoma; hedgehog inhibitors; immunotherapy.
Conflict of interest statement
Unrestricted grant from Sun pharma for this case report publication.
Figures
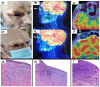
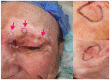
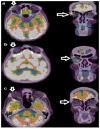
Similar articles
-
Managing Advanced Basal Cell Carcinoma: A Guide for the Dermatology Clinician.J Clin Aesthet Dermatol. 2025 Mar;18(3):21-27. J Clin Aesthet Dermatol. 2025. PMID: 40135179 Free PMC article. Review.
-
Basosquamous Carcinoma: Comprehensive Clinical and Histopathological Aspects, Novel Imaging Tools, and Therapeutic Approaches.Cells. 2023 Nov 30;12(23):2737. doi: 10.3390/cells12232737. Cells. 2023. PMID: 38067165 Free PMC article. Review.
-
Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial.Lancet Oncol. 2021 Jun;22(6):848-857. doi: 10.1016/S1470-2045(21)00126-1. Epub 2021 May 14. Lancet Oncol. 2021. PMID: 34000246 Clinical Trial.
-
The association of cemiplimab plus sonidegib for synchronous cutaneous squamous cell carcinoma and basal cell carcinoma of the head and neck: Two case reports.Front Oncol. 2023 Feb 28;13:1111146. doi: 10.3389/fonc.2023.1111146. eCollection 2023. Front Oncol. 2023. PMID: 36925925 Free PMC article.
-
European consensus-based interdisciplinary guideline for diagnosis and treatment of basal cell carcinoma-update 2023.Eur J Cancer. 2023 Oct;192:113254. doi: 10.1016/j.ejca.2023.113254. Epub 2023 Jul 28. Eur J Cancer. 2023. PMID: 37604067 Review.
Cited by
-
Successful Treatment of Cutaneous Squamous Cell Cancer with Cemiplimab-A Report of Two Cases Demonstrating the Management of Pseudoprogression and Adverse Events.J Clin Med. 2024 Jul 19;13(14):4236. doi: 10.3390/jcm13144236. J Clin Med. 2024. PMID: 39064276 Free PMC article.
References
-
- Stratigos A.J., Sekulic A., Peris K., Bechter O., Prey S., Kaatz M., Lewis K.D., Basset-Seguin N., Chang A.L.S., Dalle S., et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848–857. doi: 10.1016/S1470-2045(21)00126-1. - DOI - PubMed
-
- Guidelines CUTANEUS TUMORS NOT MELANOMA Basal Cell Carcinoma; AIOM 2021. [(accessed on 15 June 2023)]. Available online: https://www.iss.it/documents/20126/8403839/LG-AIOM-313.
-
- McDaniel B., Badri T., Steele R.B. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Basal Cell Carcinoma. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

