Herbal Compounds Dauricine and Isoliensinine Impede SARS-CoV-2 Viral Entry
- PMID: 38001915
- PMCID: PMC10669532
- DOI: 10.3390/biomedicines11112914
Herbal Compounds Dauricine and Isoliensinine Impede SARS-CoV-2 Viral Entry
Abstract
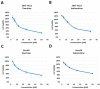
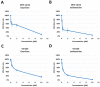
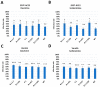
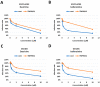
Targeting viral entry has been the focal point for the last 3 years due to the continued threat posed by SARS-CoV-2. SARS-CoV-2's entry is highly dependent on the interaction between the virus's Spike protein and host receptors. The virus's Spike protein is a key modulator of viral entry, allowing sequential cleavage of ACE2 at the S1/S2 and S2 sites, resulting in the amalgamation of membranes and subsequent entry of the virus. A Polybasic insertion (PRRAR) conveniently located at the S1/S2 site can also be cleaved by furin or by serine protease, TMPRSS2, at the cell surface. Since ACE2 and TMPRSS2 are conveniently located on the surface of host cells, targeting one or both receptors may inhibit receptor-ligand interaction. Here, we show that Dauricine and Isoliensinine, two commonly used herbal compounds, were capable of inhibiting SARS-CoV-2 viral entry by reducing Spike-ACE2 interaction but not suppressing TMPRSS2 protease activity. Further, our biological assays using pseudoviruses engineered to express Spike proteins of different variants revealed a reduction in infection rates following treatment with these compounds. The molecular modeling revealed an interconnection between R403 of Spike protein and both two compounds. Spike mutations at residue R403 are critical, and often utilized by ACE2 to gain cell access. Overall, our findings strongly suggest that Dauricine and Isoliensinine are effective in blocking Spike-ACE2 interaction and may serve as effective therapeutic agents for targeting SARS-CoV-2's viral entry.
Keywords: COVID-19; SARS-CoV-2; pandemic; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Distinctive Roles of Furin and TMPRSS2 in SARS-CoV-2 Infectivity.J Virol. 2022 Apr 27;96(8):e0012822. doi: 10.1128/jvi.00128-22. Epub 2022 Mar 28. J Virol. 2022. PMID: 35343766 Free PMC article.
-
SARS-CoV-2 Spike Furin Cleavage Site and S2' Basic Residues Modulate the Entry Process in a Host Cell-Dependent Manner.J Virol. 2022 Jul 13;96(13):e0047422. doi: 10.1128/jvi.00474-22. Epub 2022 Jun 9. J Virol. 2022. PMID: 35678602 Free PMC article.
-
Understanding the role of conserved proline and serine residues in the SARS-CoV-2 spike cleavage sites in the virus entry, fusion, and infectivity.3 Biotech. 2023 Oct;13(10):323. doi: 10.1007/s13205-023-03749-y. Epub 2023 Aug 30. 3 Biotech. 2023. PMID: 37663753 Free PMC article.
-
Roles of host proteases in the entry of SARS-CoV-2.Anim Dis. 2023;3(1):12. doi: 10.1186/s44149-023-00075-x. Epub 2023 Apr 25. Anim Dis. 2023. PMID: 37128508 Free PMC article. Review.
-
Targeting the viral-entry facilitators of SARS-CoV-2 as a therapeutic strategy in COVID-19.J Med Virol. 2021 Sep;93(9):5260-5276. doi: 10.1002/jmv.27019. Epub 2021 May 3. J Med Virol. 2021. PMID: 33851732 Free PMC article. Review.
Cited by
-
Dauricine: Review of Pharmacological Activity.Drug Des Devel Ther. 2024 Sep 27;18:4371-4385. doi: 10.2147/DDDT.S471352. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39355570 Free PMC article. Review.
-
Isoliensinine Induces Ferroptosis in Urothelial Carcinoma Cells via the PI3K/AKT/HIF-1α Axis: Molecular Evidence from Next-Generation Sequencing.Pharmaceuticals (Basel). 2025 Jul 6;18(7):1008. doi: 10.3390/ph18071008. Pharmaceuticals (Basel). 2025. PMID: 40732297 Free PMC article.
References
-
- Machhi J., Herskovitz J., Senan A.M., Dutta D., Nath B., Oleynikov M.D., Blomberg W.R., Meigs D.D., Hasan M., Patel M., et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020;15:359–386. doi: 10.1007/s11481-020-09944-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

