Whole Genome Expression Profiling of Semitendinosus Tendons from Children with Diplegic and Tetraplegic Cerebral Palsy
- PMID: 38001919
- PMCID: PMC10669597
- DOI: 10.3390/biomedicines11112918
Whole Genome Expression Profiling of Semitendinosus Tendons from Children with Diplegic and Tetraplegic Cerebral Palsy
Abstract
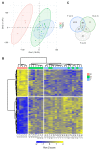
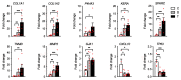
Cerebral palsy (CP) is the most common movement disorder in children, with a prevalence ranging from 1.5 to 4 per 1000 live births. CP is caused by a non-progressive lesion of the developing brain, leading to progressive alterations of the musculoskeletal system, including spasticity, often leading to the development of fixed contractures, necessitating tendon lengthening surgery. Total RNA-sequencing analysis was performed on semitendinosus tendons from diplegic and tetraplegic CP patients subjected to tendon lengthening surgery compared to control patients undergoing anterior cruciate ligament reconstructive surgery. Tetraplegic CP patients showed increased expression of genes implicated in collagen synthesis and extracellular matrix (ECM) turnover, while only minor changes were observed in diplegic CP patients. In addition, tendons from tetraplegic CP patients showed an enrichment for upregulated genes involved in vesicle-mediated transport and downregulated genes involved in cytokine and apoptotic signaling. Overall, our results indicate increased ECM turnover with increased net synthesis of collagen in tetraplegic CP patients without activation of inflammatory and apoptotic pathways, similar to observations in athletes where ECM remodeling results in increased tendon stiffness and tensile strength. Nevertheless, the resulting increased tendon stiffness is an important issue in clinical practice, where surgery is often required to restore joint mobility.
Keywords: RNA-sequencing; cerebral palsy; extracellular matrix; gene expression; tendons.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Expression profiling of genes involved in collagen turnover in tendons from cerebral palsy patients.Connect Tissue Res. 2009;50(3):203-8. doi: 10.1080/03008200802613630. Connect Tissue Res. 2009. PMID: 19444761
-
Tendon structure and extracellular matrix components are affected by spasticity in cerebral palsy patients.Muscles Ligaments Tendons J. 2013 May 21;3(1):42-50. doi: 10.11138/mltj/2013.3.1.042. Print 2013 Jan. Muscles Ligaments Tendons J. 2013. PMID: 23885344 Free PMC article.
-
Muscle and tendon lengthening behaviour of the medial gastrocnemius during ankle joint rotation in children with cerebral palsy.Exp Physiol. 2018 Oct;103(10):1367-1376. doi: 10.1113/EP087053. Epub 2018 Sep 13. Exp Physiol. 2018. PMID: 30091806
-
What causes increased passive stiffness of plantarflexor muscle-tendon unit in children with spastic cerebral palsy?Eur J Appl Physiol. 2019 Oct;119(10):2151-2165. doi: 10.1007/s00421-019-04208-4. Epub 2019 Aug 30. Eur J Appl Physiol. 2019. PMID: 31468174 Review.
-
Muscle-tendon unit in children with cerebral palsy.Dev Med Child Neurol. 2021 Aug;63(8):908-913. doi: 10.1111/dmcn.14807. Epub 2021 Jan 10. Dev Med Child Neurol. 2021. PMID: 33426691 Review.
Cited by
-
Effects of various exercise interventions on motor function in cerebral palsy patients: a systematic review and network meta-analysis.Neurol Sci. 2024 Dec;45(12):5915-5927. doi: 10.1007/s10072-024-07741-z. Epub 2024 Aug 27. Neurol Sci. 2024. PMID: 39190170
-
Identification of potential biomarkers for cerebral palsy and the development of prediction models.Exp Biol Med (Maywood). 2024 Jul 9;249:10101. doi: 10.3389/ebm.2024.10101. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 39045601 Free PMC article.
References
-
- Vogel K.G. What happens when tendons bend and twist? Proteoglycans. J. Musculoskelet. Neuronal Interact. 2004;4:202–203. - PubMed
-
- Dunkman A.A., Buckley M.R., Mienaltowski M.J., Adams S.M., Thomas S.J., Satchell L., Kumar A., Pathmanathan L., Beason D.P., Iozzo R.V., et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. doi: 10.1016/j.matbio.2012.11.005. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

