The Effects of Age on Prostatic Responses to Oxytocin and the Effects of Antagonists
- PMID: 38001957
- PMCID: PMC10669827
- DOI: 10.3390/biomedicines11112956
The Effects of Age on Prostatic Responses to Oxytocin and the Effects of Antagonists
Abstract
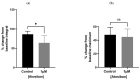
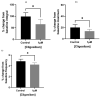
Benign prostatic hyperplasia (BPH) is an age-related enlargement of the prostate with urethral obstruction that predominantly affects the middle-aged and older male population, resulting in disruptive lower urinary tract symptoms (LUTS), thus creating a profound impact on an individual's quality of life. The development of LUTS may be linked to overexpression of oxytocin receptors (OXTR), resulting in increased baseline myogenic tone within the prostate. Thus, it is hypothesised that targeting OXTR using oxytocin receptor antagonists (atosiban, cligosiban, and β-Mercapto-β,β-cyclopentamethylenepropionyl1, O-Me-Tyr2, Orn8]-Oxytocin (ßMßßC)), may attenuate myogenic tone within the prostate. Organ bath and immunohistochemistry techniques were conducted on prostate tissue from young and older rats. Our contractility studies demonstrated that atosiban significantly decreased the frequency of spontaneous contractions within the prostate of young rats (**** p < 0.0001), and cligosiban (* p < 0.05), and ßMßßC (**** p < 0.0001) in older rats. Additionally, immunohistochemistry findings revealed that nuclear-specific OXTR was predominantly expressed within the epithelium of the prostate of both young (*** p < 0.001) and older rats (**** p < 0.0001). In conclusion, our findings indicate that oxytocin is a key modulator of prostate contractility, and targeting OXTR is a promising avenue in the development of novel BPH drugs.
Keywords: BPH; LUTS; oxytocin; oxytocin receptor antagonists.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Briganti A., Capitanio U., Suardi N., Gallina A., Salonia A., Bianchi M., Tutolo M., Di Girolamo V., Guazzoni G., Rigatti P. Benign prostatic hyperplasia and its aetiologies. Eur. Urol. Suppl. 2009;8:865–871. doi: 10.1016/j.eursup.2009.11.002. - DOI
-
- Irwin D.E., Milsom I., Hunskaar S., Reilly K., Kopp Z., Herschorn S., Coyne K., Kelleher C., Hampel C., Artibani W. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur. Urol. 2006;50:1306–1315. doi: 10.1016/j.eururo.2006.09.019. - DOI - PubMed
LinkOut - more resources
Full Text Sources

