Therapeutic Potential of Antioxidants and Hybrid TEMPOL Derivatives in Ocular Neurodegenerative Diseases: A Glimpse into the Future
- PMID: 38001960
- PMCID: PMC10669210
- DOI: 10.3390/biomedicines11112959
Therapeutic Potential of Antioxidants and Hybrid TEMPOL Derivatives in Ocular Neurodegenerative Diseases: A Glimpse into the Future
Abstract
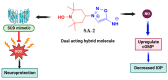
Reactive oxygen species play a significant role in the pathogenesis of various ocular neurodegenerative diseases especially glaucoma, age-related macular degeneration (AMD), and ocular ischemic stroke. Increased oxidative stress and the accumulation of ROS have been implicated in the progression of these diseases. As a result, there has been growing interest in exploring potential therapeutic and prophylactic strategies involving exogenous antioxidants. In recent years, there have been significant advancements in the development of synthetic therapeutic antioxidants for targeting reactive oxygen species (ROS) in neurodegenerative diseases. One area of focus has been the development of hybrid TEMPOL derivatives. In the context of ocular diseases, the application of next-generation hybrid TEMPOL antioxidants may offer new avenues for neuroprotection. By targeting ROS and reducing oxidative stress in the retina and optic nerve, these compounds have the potential to preserve retinal ganglion cells and trabecular meshwork and protect against optic nerve damage, mitigating irreversible blindness associated with these diseases. This review seeks to highlight the potential impact of hybrid TEMPOL antioxidants and their derivatives on ocular neurodegenerative disorders.
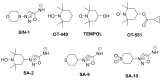
Keywords: SA-10; SA-2; SA-9; TEMPOL derivatives; glaucoma; hybrid small molecules; neuroprotection; nitric oxide; oxidative stress; reactive oxygen species; retina ganglion cells; trabecular meshwork.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Current trends in targeting the oxidative stress in glaucoma (Review).Eur J Ophthalmol. 2024 Mar;34(2):328-337. doi: 10.1177/11206721231214297. Epub 2023 Nov 17. Eur J Ophthalmol. 2024. PMID: 37974458 Review.
-
Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases.Mutat Res. 2013 Apr-Jun;752(2):153-171. doi: 10.1016/j.mrrev.2013.01.001. Epub 2013 Jan 19. Mutat Res. 2013. PMID: 23337404 Review.
-
Antioxidants and neurodegenerative eye disease.Crit Rev Food Sci Nutr. 2024 Sep;64(26):9672-9690. doi: 10.1080/10408398.2023.2215865. Epub 2023 Jun 13. Crit Rev Food Sci Nutr. 2024. PMID: 37312562 Review.
-
Polydopamine nanoparticles attenuate retina ganglion cell degeneration and restore visual function after optic nerve injury.J Nanobiotechnology. 2021 Dec 20;19(1):436. doi: 10.1186/s12951-021-01199-3. J Nanobiotechnology. 2021. PMID: 34930292 Free PMC article.
-
Novel Thiol Containing Hybrid Antioxidant-Nitric Oxide Donor Small Molecules for Treatment of Glaucoma.Antioxidants (Basel). 2021 Apr 8;10(4):575. doi: 10.3390/antiox10040575. Antioxidants (Basel). 2021. PMID: 33917924 Free PMC article.
Cited by
-
Tert-butyl hydroperoxide induces trabecular meshwork cells injury through ferroptotic cell death.J Cell Commun Signal. 2024 Aug 28;18(3):e12050. doi: 10.1002/ccs3.12050. eCollection 2024 Sep. J Cell Commun Signal. 2024. PMID: 39524143 Free PMC article.
-
The Role and Mechanism of Nicotinamide Riboside in Oxidative Damage and a Fibrosis Model of Trabecular Meshwork Cells.Transl Vis Sci Technol. 2024 Mar 1;13(3):24. doi: 10.1167/tvst.13.3.24. Transl Vis Sci Technol. 2024. PMID: 38546981 Free PMC article.
-
Platelet hyperreactivity and frailty in a mouse model of Alzheimer's disease are prevented by anti-oxidant treatment.Geroscience. 2025 Jun 3. doi: 10.1007/s11357-025-01710-w. Online ahead of print. Geroscience. 2025. PMID: 40459816
-
Nitroxides: Chemistry, Antioxidant Properties, and Biomedical Applications.Molecules. 2025 May 14;30(10):2159. doi: 10.3390/molecules30102159. Molecules. 2025. PMID: 40430331 Free PMC article. Review.
-
Immunomodulation by 4-Hydroxy-TEMPO (TEMPOL) and Dimethyl Fumarate (DMF) After Ventral Root Crush (VRC) in C57BL/6J Mice: A Flow Cytometry Analysis.Biology (Basel). 2025 Apr 25;14(5):473. doi: 10.3390/biology14050473. Biology (Basel). 2025. PMID: 40427663 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

