Clinical Applicability of Tissue Polypeptide Antigen and CA-125 in Gynecological Malignancies
- PMID: 38001961
- PMCID: PMC10669758
- DOI: 10.3390/biomedicines11112960
Clinical Applicability of Tissue Polypeptide Antigen and CA-125 in Gynecological Malignancies
Abstract
Background: Nowadays there still is no sufficient screening tool for ovarian and uterine cancer.
Objective: The current study aimed to investigate whether cancer antigen 125 (CA-125), tissue polypeptide antigen (TPA) or the combination of both markers are able to act as screening tools for ovarian or uterine cancer.
Methods: A total of 275 blood samples from different cohorts (ovarian cancer, uterine cancer, benign control group) were prospectively drawn and analyzed.
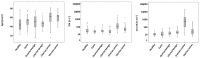
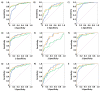
Results: Established biomarkers TPA and CA-125 showed elevated serum concentrations in patients with malignant tumors as compared to healthy women and women with benign diseases. In ROC curve analyses, both biomarkers were well able to discriminate between malignant and healthy, benign or overall non-malignant cases in the whole sample, with AUCs of 0.842 and above. While TPA was the best diagnostic marker in patients with uterine cancer, CA 125 was the best in patients with ovarian cancer.
Conclusions: TPA and CA-125 both showed promising results for the detection of gynecologic malignancies. The combination of CA-125 and TPA did not improve sensitivity in comparison to single markers.
Keywords: CA 125; TPA; diagnosis; ovarian cancer; tumor marker; uterine cancer.
Conflict of interest statement
L.S., C.M.D., K.M.E.G., C.N., A.B.A.R. and M.R.M. report no conflicts of interest. S.H. has received research funding or honoraria from EU WCT EVI MAP, Roche, BMS, Merck, Sysmex, Thermo, Volition, Instand and is the founder of SFZ BioCoDE and CEBIO.
Figures
Similar articles
-
[Tissue polypeptide antigen as a tumor marker for gynecologic malignancies].Nihon Sanka Fujinka Gakkai Zasshi. 1985 Sep;37(9):1799-805. Nihon Sanka Fujinka Gakkai Zasshi. 1985. PMID: 4056529 Japanese.
-
[The clinical significance of tissue polypeptide antigen (TPA) in the patients with gynecologic tumor].Nihon Sanka Fujinka Gakkai Zasshi. 1984 May;36(5):779-84. Nihon Sanka Fujinka Gakkai Zasshi. 1984. PMID: 6736725 Japanese.
-
Evaluation of seven different tumour markers for the establishment of tumour marker panels in gynecologic malignancies.Eur J Gynaecol Oncol. 1989;10(6):395-405. Eur J Gynaecol Oncol. 1989. PMID: 2627971
-
Ovarian cancer screening. The use of serial complementary tumor markers to improve sensitivity and specificity for early detection.Cancer. 1995 Nov 15;76(10 Suppl):2092-6. doi: 10.1002/1097-0142(19951115)76:10+<2092::aid-cncr2820761331>3.0.co;2-t. Cancer. 1995. PMID: 8635006 Review.
-
Benefits and limitations of ultrasonographic evaluation of uterine adnexal lesions in early detection of ovarian cancer.Clin Exp Obstet Gynecol. 2004;31(2):85-98. Clin Exp Obstet Gynecol. 2004. PMID: 15266758 Review.
Cited by
-
Immunogenic Biomarkers HMGB1 and sRAGE Are Potential Diagnostic Tools for Ovarian Malignancies.Cancers (Basel). 2023 Oct 20;15(20):5081. doi: 10.3390/cancers15205081. Cancers (Basel). 2023. PMID: 37894448 Free PMC article.
References
-
- Stewart B.W., Wild C. World Cancer Report 2014. International Agency for Research on Cancer; WHO Press, World Health Organization; Lyon, France: Geneva, Switzerland: 2014.
-
- Sölétormos G., Duffy M.J., Othman Abu Hassan S., Verheijen R.H.M., Tholander B., Bast R.C., Gaarenstroom K.N., Sturgeon C.M., Bonfrer J.M., Petersen P.H., et al. Clinical Use of Cancer Biomarkers in Epithelial Ovarian Cancer: Updated Guidelines from the European Group on Tumor Markers. Int. J. Gynecol. Cancer. 2016;26:43–51. doi: 10.1097/IGC.0000000000000586. - DOI - PMC - PubMed
-
- Morgan R.J., Armstrong D.K., Alvarez R.D., Bakkum-Gamez J.N., Behbakht K., Chen L., Copeland L., Crispens M.A., DeRosa M., Dorigo O., et al. Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2016;14:1134–1163. doi: 10.6004/jnccn.2016.0122. - DOI - PubMed
-
- Terry K.L., Schock H., Fortner R.T., Hüsing A., Fichorova R.N., Yamamoto H.S., Vitonis A.F., Johnson T., Overvad K., Tjønneland A., et al. A Prospective Evaluation of Early Detection Biomarkers for Ovarian Cancer in the European EPIC Cohort. Clin. Cancer. Res. 2016;22:4664–4675. doi: 10.1158/1078-0432.CCR-16-0316. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

