The RNA-Binding Function of Ribosomal Proteins and Ribosome Biogenesis Factors in Human Health and Disease
- PMID: 38001969
- PMCID: PMC10669870
- DOI: 10.3390/biomedicines11112969
The RNA-Binding Function of Ribosomal Proteins and Ribosome Biogenesis Factors in Human Health and Disease
Abstract
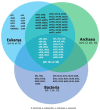
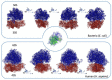
The ribosome is a macromolecular complex composed of RNA and proteins that interact through an integrated and interconnected network to preserve its ancient core activities. In this review, we emphasize the pivotal role played by RNA-binding proteins as a driving force in the evolution of the current form of the ribosome, underscoring their importance in ensuring accurate protein synthesis. This category of proteins includes both ribosomal proteins and ribosome biogenesis factors. Impairment of their RNA-binding activity can also lead to ribosomopathies, which is a group of disorders characterized by defects in ribosome biogenesis that are detrimental to protein synthesis and cellular homeostasis. A comprehensive understanding of these intricate processes is essential for elucidating the mechanisms underlying the resulting diseases and advancing potential therapeutic interventions.
Keywords: DBA; RNA-binding proteins; SBDS; ribosomal RNA; ribosomal origins and evolution; ribosomopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ribosomopathies: how a common root can cause a tree of pathologies.Dis Model Mech. 2015 Sep;8(9):1013-26. doi: 10.1242/dmm.020529. Dis Model Mech. 2015. PMID: 26398160 Free PMC article. Review.
-
Ribosomal proteins and human diseases: molecular mechanisms and targeted therapy.Signal Transduct Target Ther. 2021 Aug 30;6(1):323. doi: 10.1038/s41392-021-00728-8. Signal Transduct Target Ther. 2021. PMID: 34462428 Free PMC article. Review.
-
Ribosomopathies: There's strength in numbers.Science. 2017 Nov 3;358(6363):eaan2755. doi: 10.1126/science.aan2755. Science. 2017. PMID: 29097519 Review.
-
Cancer Biogenesis in Ribosomopathies.Cells. 2019 Mar 11;8(3):229. doi: 10.3390/cells8030229. Cells. 2019. PMID: 30862070 Free PMC article. Review.
-
Inhibition of Ribosome Assembly and Ribosome Translation Has Distinctly Different Effects on Abundance and Paralogue Composition of Ribosomal Protein mRNAs in Saccharomyces cerevisiae.mSystems. 2023 Feb 23;8(1):e0109822. doi: 10.1128/msystems.01098-22. Epub 2023 Jan 18. mSystems. 2023. PMID: 36651729 Free PMC article.
Cited by
-
Ribosome Biogenesis and Cancer: Insights into NOB1 and PNO1 Mechanisms.Curr Pharm Des. 2024;30(37):2911-2921. doi: 10.2174/0113816128301870240730071910. Curr Pharm Des. 2024. PMID: 39143880 Review.
-
RNA-binding protein transcripts as potential biomarkers for detecting Primary Sclerosing Cholangitis and for predicting its progression to Cholangiocarcinoma.Front Mol Biosci. 2024 Jun 6;11:1388294. doi: 10.3389/fmolb.2024.1388294. eCollection 2024. Front Mol Biosci. 2024. PMID: 38903178 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

