A Microsurgical Arteriovenous Malformation Model on Saphenous Vessels in the Rat
- PMID: 38001970
- PMCID: PMC10669800
- DOI: 10.3390/biomedicines11112970
A Microsurgical Arteriovenous Malformation Model on Saphenous Vessels in the Rat
Abstract
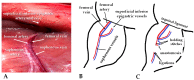
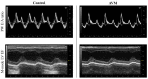
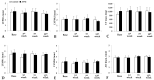
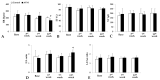
Arteriovenous malformation (AVM) is an anomaly of blood vessel formation. Numerous models have been established to understand the nature of AVM. These models have limitations in terms of the diameter of the vessels used and the impact on the circulatory system. Our goal was to establish an AVM model that does not cause prompt and significant hemodynamic and cardiac alterations but is feasible for follow-up of the AVM's progression. Sixteen female rats were randomly divided into sham-operated and AVM groups. In the AVM group, the saphenous vein and artery were interconnected using microsurgical techniques. The animals were followed up for 12 weeks. Anastomosis patency and the structural and hemodynamic changes of the heart were monitored. The hearts and vessels were histologically analyzed. During the follow-up period, shunts remained unobstructed. Systolic, diastolic, mean arterial pressure, and heart rate values slightly and non-significantly decreased in the AVM group. Echocardiogram results indicated minor systolic function impact, with slight and insignificant changes in aortic pressure and blood velocity, and minimal left ventricular wall enlargement. The small-caliber saphenous AVM model does not cause acute hemodynamic changes. Moderate but progressive alterations and venous dilatation confirmed AVM-like features. The model seems to be suitable for studying further the progression, enlargement, or destabilization of AVM.
Keywords: arteriovenous malformation; arteriovenous shunt; hemodynamics; microsurgical model; vascular remodelling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Minor micro-rheological alterations in the presence of an artificial saphenous arteriovenous shunt, as an arteriovenous malformation model in the rat.Clin Hemorheol Microcirc. 2024;87(1):27-37. doi: 10.3233/CH-231825. Clin Hemorheol Microcirc. 2024. PMID: 38250764
-
Cerebral arteriovenous malformation venous stenosis is associated with hemodynamic changes at the draining vein-venous sinus junction.Med Hypotheses. 2019 Feb;123:86-88. doi: 10.1016/j.mehy.2019.01.003. Epub 2019 Jan 7. Med Hypotheses. 2019. PMID: 30696602 Free PMC article.
-
Direct continuous measurement of draining vein pressure during Onyx embolization in a swine arteriovenous malformation model.J Neurointerv Surg. 2015 Jan;7(1):62-6. doi: 10.1136/neurintsurg-2013-011066. Epub 2014 Jan 17. J Neurointerv Surg. 2015. PMID: 24443412
-
The combined management of cerebral arteriovenous malformations. Experience with 100 cases and review of the literature.Acta Neurochir (Wien). 1993;123(3-4):101-12. doi: 10.1007/BF01401864. Acta Neurochir (Wien). 1993. PMID: 8237486 Review.
-
Venous malformation serving as the draining vein of an adjoining arteriovenous malformation. Case report and review of the literature.Surg Neurol. 2001 Sep;56(3):170-4. doi: 10.1016/s0090-3019(01)00457-8. Surg Neurol. 2001. PMID: 11597644 Review.
Cited by
-
Meta-analysis of arterial spin labeling MRI to identify residual cerebral arteriovenous malformations after treatment.BMC Med Imaging. 2025 Apr 18;25(1):127. doi: 10.1186/s12880-025-01668-3. BMC Med Imaging. 2025. PMID: 40251605 Free PMC article.
References
-
- Halim A.X., Johnston S.C., Singh V., McCulloch C.E., Bennett J.P., Achrol A.S., Sidney S., Young W.L. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke. 2004;35:1697–1702. doi: 10.1161/01.STR.0000130988.44824.29. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

