JSI-124 Induces Cell Cycle Arrest and Regulates the Apoptosis in Glioblastoma Cells
- PMID: 38001999
- PMCID: PMC10669163
- DOI: 10.3390/biomedicines11112999
JSI-124 Induces Cell Cycle Arrest and Regulates the Apoptosis in Glioblastoma Cells
Abstract
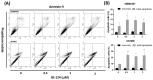
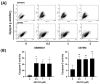
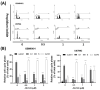
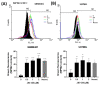
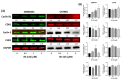
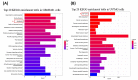
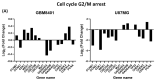
Cucurbitacin I (JSI-124), derived from Cucurbitaceae, has shown the potential to induce apoptosis and cell cycle arrest in some cancer cells. However, the effect of JSI-124 on glioblastoma multiforme (GBM) cell cycle and apoptosis is still unclear. Our investigation revealed that JSI-124 effectively reduced cell viability in GBM cells, leading to apoptosis and increased caspase-3 activity. Intriguingly, JSI-124 caused the accumulation of G2/M phase to regulate cell cycle, confirmed by MPM-2 staining and increased protein synthesis during mitosis by mitotic index analysis. Western blot analysis found that JSI-124 affected the progression of G2/M arrest by downregulating the CDK1 and upregulating the cyclinB1, suggesting that JSI-124 disrupted the formation and function of the cyclin B1/CDK1 complex in GBM8401 and U87MG cells. However, we found the JSI-124-regulated cell cycle G2/M and apoptosis-relative gene in GBM8401 and U87MG cells by NGS data analysis. Notably, we found that the GBM8401 and U87MG cells observed regulation of apoptosis and cell-cycle-related signaling pathways. Taken together, JSI-124 exhibited the ability to induce G2/M arrest, effectively arresting the cell cycle at critical stages. This arrest is accompanied by the initiation of apoptosis, highlighting the dual mechanism of action of JSI-124. Collectively, our findings emphasize that JSI-124 holds potential as a therapeutic agent for GBM by impeding cell cycle progression, inhibiting cell proliferation, and promoting apoptosis. As demonstrated by our in vitro experiments, these effects are mediated through modulation of key molecular targets.
Keywords: apoptosis; cell cycle; cucurbitacin I; glioblastoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
JSI-124 inhibits glioblastoma multiforme cell proliferation through G(2)/M cell cycle arrest and apoptosis augment.Cancer Biol Ther. 2008 Aug;7(8):1243-9. doi: 10.4161/cbt.7.8.6263. Epub 2008 Aug 10. Cancer Biol Ther. 2008. PMID: 18487947
-
Induction of Mitosis Delay and Apoptosis by CDDO-TFEA in Glioblastoma Multiforme.Front Pharmacol. 2021 Nov 8;12:756228. doi: 10.3389/fphar.2021.756228. eCollection 2021. Front Pharmacol. 2021. PMID: 34858180 Free PMC article.
-
Cucurbitacin-I (JSI-124) activates the JNK/c-Jun signaling pathway independent of apoptosis and cell cycle arrest in B leukemic cells.BMC Cancer. 2011 Jun 24;11:268. doi: 10.1186/1471-2407-11-268. BMC Cancer. 2011. PMID: 21702955 Free PMC article.
-
Foretinib induces G2/M cell cycle arrest, apoptosis, and invasion in human glioblastoma cells through c-MET inhibition.Cancer Chemother Pharmacol. 2021 Jun;87(6):827-842. doi: 10.1007/s00280-021-04242-0. Epub 2021 Mar 10. Cancer Chemother Pharmacol. 2021. PMID: 33688998
-
3-O-acetyl-11-keto-β-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase.J Exp Clin Cancer Res. 2018 Jul 3;37(1):132. doi: 10.1186/s13046-018-0805-4. J Exp Clin Cancer Res. 2018. PMID: 29970196 Free PMC article.
References
-
- Rock K., McArdle O., Forde P., Dunne M., Fitzpatrick D., O’Neill B., Faul C. A clinical review of treatment outcomes in glioblastoma multiforme--the validation in a non-trial population of the results of a randomised Phase III clinical trial: Has a more radical approach improved survival? Br. J. Radiol. 2012;85:e729–e733. doi: 10.1259/bjr/83796755. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

