Nerve Growth Factor, Antimicrobial Peptides and Chemotherapy: Glioblastoma Combination Therapy to Improve Their Efficacy
- PMID: 38002009
- PMCID: PMC10669874
- DOI: 10.3390/biomedicines11113009
Nerve Growth Factor, Antimicrobial Peptides and Chemotherapy: Glioblastoma Combination Therapy to Improve Their Efficacy
Abstract
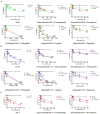
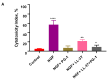
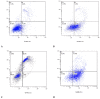
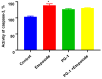
Glioblastoma (GBM) is an aggressive and lethal malignancy of the central nervous system with a median survival rate of 15 months. We investigated the combined anticancer effects of nerve growth factor (NGF), cathelicidin (LL-37), and protegrin-1 (PG-1) with chemotherapy (temozolomide, doxorubicin, carboplatin, cisplatin, and etoposide) in the glioblastoma U251 cell line to overcome the limitations of conventional chemotherapy and to guarantee specific treatments to succeed. The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to study cell viability and to determine the cytotoxic effects of NGF, LL-37, and PG-1 and their combination with chemotherapy in U251 cells. Synergism or antagonism was determined using the combination index (CI) method. Caspase-3 activity was evaluated spectrophotometrically using a caspase-3 activity assay kit. Apoptosis was analyzed with flow cytometry using propidium iodide (PI) and YO-PRO-1. NGF and the peptides showed a strong cytotoxic effect on U251 glioma cells in the MTT test (IC50 0.0214, 3.1, and 26.1 μM, respectively) compared to chemotherapy. The combination of PG-1 + etoposide had a synergistic effect on apoptosis of U251 glioma cells. It should be noted that the cells were in the early and late stages of apoptosis, respectively, compared with the control cells. The caspase-3 activation analysis revealed that the caspase-3 level was not significantly (p > 0.05) increased in U251 cells following PG-1 with etoposide treatment compared with that in the untreated cells, suggesting that the combination of PG-1 and etoposide may induce caspase-independent apoptosis in U251 cells. NGF, LL-37, and PG-1 represent promising drug candidates as the treatment regimen for GBM. Furthermore, the synergistic efficacy of the combined protocol using PG-1 and etoposide may overcome some of the typical limitations of the conventional therapeutic protocols, thus representing a promising approach for GBM therapy.
Keywords: antimicrobial peptides; chemotherapy; glioblastoma; nerve growth factor; synergistic effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Glioblastoma Multiforme: Sensitivity to Antimicrobial Peptides LL-37 and PG-1, and Their Combination with Chemotherapy for Predicting the Overall Survival of Patients.Pharmaceutics. 2024 Sep 22;16(9):1234. doi: 10.3390/pharmaceutics16091234. Pharmaceutics. 2024. PMID: 39339270 Free PMC article.
-
Expression of molecular markers and synergistic anticancer effects of chemotherapy with antimicrobial peptides on glioblastoma cells.Cancer Chemother Pharmacol. 2024 May;93(5):455-469. doi: 10.1007/s00280-023-04622-8. Epub 2024 Jan 27. Cancer Chemother Pharmacol. 2024. PMID: 38280033
-
Anticancer Effect of Cathelicidin LL-37, Protegrin PG-1, Nerve Growth Factor NGF, and Temozolomide: Impact on the Mitochondrial Metabolism, Clonogenic Potential, and Migration of Human U251 Glioma Cells.Molecules. 2022 Aug 5;27(15):4988. doi: 10.3390/molecules27154988. Molecules. 2022. PMID: 35956937 Free PMC article.
-
In Vitro Evaluation of the Cytotoxic Effect of Streptococcus pyogenes Strains, Protegrin PG-1, Cathelicidin LL-37, Nerve Growth Factor and Chemotherapy on the C6 Glioma Cell Line.Molecules. 2022 Jan 17;27(2):569. doi: 10.3390/molecules27020569. Molecules. 2022. PMID: 35056889 Free PMC article.
-
Treatment of relapsed aggressive lymphomas: regimens with and without high-dose therapy and stem cell rescue.Cancer Chemother Pharmacol. 2002 May;49 Suppl 1:S13-20. doi: 10.1007/s00280-002-0447-1. Epub 2002 Apr 12. Cancer Chemother Pharmacol. 2002. PMID: 12042984 Review.
Cited by
-
Glioblastoma Multiforme: Sensitivity to Antimicrobial Peptides LL-37 and PG-1, and Their Combination with Chemotherapy for Predicting the Overall Survival of Patients.Pharmaceutics. 2024 Sep 22;16(9):1234. doi: 10.3390/pharmaceutics16091234. Pharmaceutics. 2024. PMID: 39339270 Free PMC article.
References
-
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global cancer observatory: Cancer today. Lyon Fr. Int. Agency Res. Cancer. 2018;3:2019.
-
- Nabors L.B., Portnow J., Ahluwalia M., Baehring J., Brem H., Brem S., Butowski N., Campian J.L., Clark S.W., Fabiano A.J., et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN. 2020;18:1537–1570. doi: 10.6004/jnccn.2020.0052. - DOI - PubMed
-
- Stupp R., Taillibert S., Kanner A., Read W., Steinberg D., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

