The Ultraviolet Irradiation of Keratinocytes Induces Ectopic Expression of LINE-1 Retrotransposon Machinery and Leads to Cellular Senescence
- PMID: 38002016
- PMCID: PMC10669206
- DOI: 10.3390/biomedicines11113017
The Ultraviolet Irradiation of Keratinocytes Induces Ectopic Expression of LINE-1 Retrotransposon Machinery and Leads to Cellular Senescence
Abstract
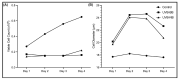
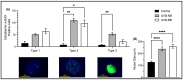
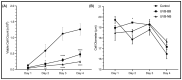
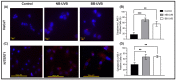
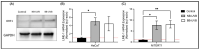
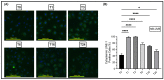
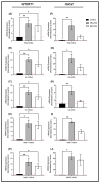
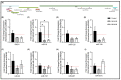
Retrotransposons have played an important role in evolution through their transposable activity. The largest and the only currently active human group of mobile DNAs are the LINE-1 retrotransposons. The ectopic expression of LINE-1 has been correlated with genomic instability. Narrow-band ultraviolet B (NB-UVB) and broad-band ultraviolet B (BB-UVB) phototherapy is commonly used for the treatment of dermatological diseases. UVB exposure is carcinogenic and can lead, in keratinocytes, to genomic instability. We hypothesize that LINE-1 reactivation occurs at a high rate in response to UVB exposure on the skin, which significantly contributes to genomic instability and DNA damage leading to cellular senescence and photoaging. Immortalized N/TERT1 and HaCaT human keratinocyte cell lines were irradiated in vitro with either NB-UVB or BB-UVB. Using immunofluorescence and Western blotting, we confirmed UVB-induced protein expression of LINE-1. Using RT-qPCR, we measured the mRNA expression of LINE-1 and senescence markers that were upregulated after several NB-UVB exposures. Selected miRNAs that are known to bind LINE-1 mRNA were measured using RT-qPCR, and the expression of miR-16 was downregulated with UVB exposure. Our findings demonstrate that UVB irradiation induces LINE-1 reactivation and DNA damage in normal keratinocytes along with the associated upregulation of cellular senescence markers and change in miR-16 expression.
Keywords: BB-UVB; HaCaT; N/TERT1; NB-UVB; cellular senescence; genomic instability; keratinocytes; long interspersed nucleotide element-1 (LINE-1); miR-16; microRNAs; phototherapy.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
NB-UVB irradiation downregulates keratin-17 expression in keratinocytes by inhibiting the ERK1/2 and STAT3 signaling pathways.Arch Dermatol Res. 2018 Mar;310(2):147-156. doi: 10.1007/s00403-018-1812-1. Epub 2018 Jan 18. Arch Dermatol Res. 2018. PMID: 29349514
-
Narrow-band UVB induces apoptosis in human keratinocytes.J Photochem Photobiol B. 2006 Feb 1;82(2):132-9. doi: 10.1016/j.jphotobiol.2005.08.011. Epub 2005 Nov 23. J Photochem Photobiol B. 2006. PMID: 16309917
-
Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes.Nutrients. 2016 Jul 29;8(8):464. doi: 10.3390/nu8080464. Nutrients. 2016. PMID: 27483310 Free PMC article.
-
MiR-23a regulates DNA damage repair and apoptosis in UVB-irradiated HaCaT cells.J Dermatol Sci. 2013 Jan;69(1):68-76. doi: 10.1016/j.jdermsci.2012.10.014. Epub 2012 Oct 30. J Dermatol Sci. 2013. PMID: 23158364
-
Effects of narrow band UVB (311 nm) irradiation on epidermal cells.Int J Mol Sci. 2013 Apr 17;14(4):8456-66. doi: 10.3390/ijms14048456. Int J Mol Sci. 2013. PMID: 23594996 Free PMC article. Review.
Cited by
-
Photoaging: UV radiation-induced cGAS-STING signaling promotes the aging process in skin by remodeling the immune network.Biogerontology. 2025 Jun 20;26(4):123. doi: 10.1007/s10522-025-10268-1. Biogerontology. 2025. PMID: 40542276 Free PMC article. Review.
-
Quercetin ameliorates senescence and promotes osteogenesis of BMSCs by suppressing the repetitive element‑triggered RNA sensing pathway.Int J Mol Med. 2025 Jan;55(1):4. doi: 10.3892/ijmm.2024.5445. Epub 2024 Oct 25. Int J Mol Med. 2025. PMID: 39450556 Free PMC article.
References
-
- De Gruijl F.R. Methods in Enzymology. Volume 319. Elsevier; Amsterdam, The Netherlands: 2000. [33] Photocarcinogenesis: UVA vs. UVB; pp. 359–366. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

