Exploring Differences in Pharmacometrics of Rabeprazole between Genders via Population Pharmacokinetic-Pharmacodynamic Modeling
- PMID: 38002021
- PMCID: PMC10669052
- DOI: 10.3390/biomedicines11113021
Exploring Differences in Pharmacometrics of Rabeprazole between Genders via Population Pharmacokinetic-Pharmacodynamic Modeling
Abstract
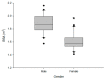
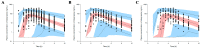
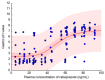
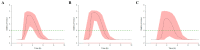
Rabeprazole is a proton pump inhibitor that inhibits gastric acid production and increases gastric pH; it is widely used clinically as a treatment option for gastritis and gastric ulcers. However, information on the inter-individual variability of rabeprazole pharmacometrics, which is a key element in establishing its scientific clinical use, is still lacking. Particularly, the differences in pharmacokinetics between genders and the degree of variation in pharmacodynamics have not been clearly identified. Thus, the main purpose of this study was to explore any differences in rabeprazole pharmacokinetics between genders and to quantitatively predict and compare the effects of any differences in pharmacokinetics between genders on known pharmacodynamics using population pharmacokinetic-pharmacodynamic modeling. To compare pharmacokinetics and modeling data between genders, bioequivalence results were used simultaneously on healthy Korean men and women using the physiological and biochemical parameters derived from each individual. Pharmacodynamic modeling was performed based on the data of previously reported gastric pH changes in response to rabeprazole plasma concentrations, which was co-linked to the central compartmental bioavailable concentration in the population pharmacokinetic model. There was no significant difference in the level of rabeprazole exposure and elimination of plasma between genders following oral administration of 10 mg enteric-coated rabeprazole tablets; however, there was a clear delay in absorption in women compared to men. Additionally, a comparison of pharmacokinetic parameters normalized to body weight between genders showed that the maximum plasma concentrations were significantly higher in women than in men, again suggesting gender differences in rabeprazole absorption. The population pharmacokinetic profiles for rabeprazole were described using a three-sequential multi-absorption with lag time (Tlag) two-compartment model, whereas body surface area and gender were explored as effective covariates for absorption rate constant and Tlag, respectively. The effect of increased gastric pH due to plasma exposure to rabeprazole was explained using the Sigmoid Emax model, with the baseline as a direct response. The significantly longer rabeprazole Tlag in females delayed the onset of an effect by an average of 1.58 times (2.02-3.20 h), yet the overall and maximum effects did not cause a significant difference within 15%. In the relative comparison of the overall efficacy of rabeprazole enteric-coated tablet administration between genders, it was predicted based on the model that males would have higher efficacy. This study will be very useful in broadening the perspective of interpreting drug diversity between individuals and narrowing the gap in knowledge related to scientific precision medicine by presenting new information on gender differences in rabeprazole pharmacometrics that had not been previously identified.
Keywords: absorption phase; gastric pH; gender differences; pharmacodynamics; population pharmacokinetic modeling; rabeprazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Is Gender an Important Factor in the Precision Medicine Approach to Levocetirizine?Pharmaceutics. 2024 Jan 21;16(1):146. doi: 10.3390/pharmaceutics16010146. Pharmaceutics. 2024. PMID: 38276516 Free PMC article.
-
Modeling population pharmacokinetics of morniflumate in healthy Korean men: extending pharmacometrics analysis to niflumic acid, its major active metabolite.Naunyn Schmiedebergs Arch Pharmacol. 2024 Feb;397(2):843-856. doi: 10.1007/s00210-023-02640-0. Epub 2023 Jul 29. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37515737
-
Population pharmacokinetics of rabeprazole and dosing recommendations for the treatment of gastroesophageal reflux disease in children aged 1-11 years.Clin Pharmacokinet. 2014 Oct;53(10):943-57. doi: 10.1007/s40262-014-0168-8. Clin Pharmacokinet. 2014. PMID: 25168707
-
Review article: relationship between the metabolism and efficacy of proton pump inhibitors--focus on rabeprazole.Aliment Pharmacol Ther. 2004 Nov;20 Suppl 6:11-9. doi: 10.1111/j.1365-2036.2004.02161.x. Aliment Pharmacol Ther. 2004. PMID: 15496214 Review.
-
Clinical pharmacology of proton pump inhibitors: what the practising physician needs to know.Drugs. 2003;63(24):2739-54. doi: 10.2165/00003495-200363240-00004. Drugs. 2003. PMID: 14664653 Review.
Cited by
-
Is Gender an Important Factor in the Precision Medicine Approach to Levocetirizine?Pharmaceutics. 2024 Jan 21;16(1):146. doi: 10.3390/pharmaceutics16010146. Pharmaceutics. 2024. PMID: 38276516 Free PMC article.
-
Exploring gender differences in pharmacokinetics of central nervous system related medicines based on a systematic review approach.Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):8311-8347. doi: 10.1007/s00210-024-03190-9. Epub 2024 Jun 8. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38850303
-
Considerations of sex in bioequivalence assessments: does sex affect pharmacokinetic variability between evaluation formulations?Eur J Clin Pharmacol. 2025 Apr;81(4):583-596. doi: 10.1007/s00228-025-03813-x. Epub 2025 Feb 25. Eur J Clin Pharmacol. 2025. PMID: 40000474
References
-
- Miwa H., Sasaki M., Furuta T., Koike T., Habu Y., Ito M., Fujiwara Y., Wada T., Nagahara A., Hongo M. Efficacy of rabeprazole on heartburn symptom resolution in patients with non-erosive and erosive gastro-oesophageal reflux disease: A multicenter study from Japan. Aliment. Pharmacol. Ther. 2007;26:69–77. doi: 10.1111/j.1365-2036.2007.03350.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

