Endocervical Adenocarcinoma Showing Microcystic, Elongated, and Fragmented (MELF) Pattern of Stromal Invasion: A Single-Institutional Analysis of 10 Cases with Comprehensive Clinicopathological Analyses and Ki-67 Immunostaining
- PMID: 38002025
- PMCID: PMC10669505
- DOI: 10.3390/biomedicines11113026
Endocervical Adenocarcinoma Showing Microcystic, Elongated, and Fragmented (MELF) Pattern of Stromal Invasion: A Single-Institutional Analysis of 10 Cases with Comprehensive Clinicopathological Analyses and Ki-67 Immunostaining
Abstract
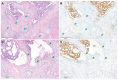
Microcystic, elongated, and fragmented (MELF) pattern of invasion has seldom been documented in endocervical adenocarcinoma (EAC). The aim of this study was to analyze the clinicopathological characteristics of EAC showing MELF pattern. We collected the clinicopathological information of 10 cases of EAC with the MELF pattern and conducted polymer-based immunostaining for Ki-67 (dilution 1:200, clone MIB-1) on these cases. Ki-67 expression was assessed using the average estimation within the hotspot method. All tumors were human papillomavirus-associated EAC with Silva pattern C. All except one tumor exceeded 3 cm in size. Five tumors involved the entire thickness of the cervical stroma, and four tumors extended into the parametrium. Lymphovascular space invasion was identified in six cases. Two patients developed metastatic recurrences in the para-aortic lymph nodes and lungs, respectively. The MELF area showed significantly lower Ki-67 labelling index than that of a conventional tumor area. We confirmed our previous observation that the MELF area displayed lower proliferative activity than the conventional tumor area of EAC. We also demonstrated that patients with EAC showing MELF pattern had several adverse clinicopathological characteristics reflecting aggressive behavior. On the other hand, since the frequencies of post-operative recurrence and disease-related mortality that occurred during the follow-up period were relatively low, further investigations are warranted to clarify the prognostic value of MELF pattern in EAC patients.
Keywords: immunohistochemistry; mesonephric-like adenocarcinoma; mismatch repair; programmed cell death-ligand 1; targeted sequencing; uterus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Usual-Type Endocervical Adenocarcinoma with a Microcystic, Elongated, and Fragmented Pattern of Stromal Invasion: A Case Report with Emphasis on Ki-67 Immunostaining and Targeted Sequencing Results.Case Rep Oncol. 2020 Nov 30;13(3):1421-1429. doi: 10.1159/000510441. eCollection 2020 Sep-Dec. Case Rep Oncol. 2020. PMID: 33442366 Free PMC article.
-
[Microcystic, elongated and fragmented invasion pattern in endometrial carcinoma: the clinicopathology analysis].Zhonghua Fu Chan Ke Za Zhi. 2018 Dec 25;53(12):811-815. doi: 10.3760/cma.j.issn.0529-567x.2018.12.003. Zhonghua Fu Chan Ke Za Zhi. 2018. PMID: 30585018 Chinese.
-
Clinicopathologic Association and Prognostic Value of MELF Pattern in Invasive Endocervical Adenocarcinoma (ECA) as Classified by IECC.Int J Gynecol Pathol. 2020 Sep;39(5):436-442. doi: 10.1097/PGP.0000000000000633. Int J Gynecol Pathol. 2020. PMID: 31517653 Free PMC article.
-
Clinicopathological and prognostic significance of the microcystic elongated and fragmented pattern in endometrial cancer: a systematic review and meta-analysis.BMJ Open. 2025 Jan 28;15(1):e092006. doi: 10.1136/bmjopen-2024-092006. BMJ Open. 2025. PMID: 39880438 Free PMC article.
-
MELF pattern of myometrial invasion and role in possible endometrial cancer diagnostic pathway: A systematic review of the literature.Eur J Obstet Gynecol Reprod Biol. 2018 Nov;230:147-152. doi: 10.1016/j.ejogrb.2018.09.036. Epub 2018 Sep 25. Eur J Obstet Gynecol Reprod Biol. 2018. PMID: 30286364
Cited by
-
Ovarian Metastasis from Human Papillomavirus-associated Usual-type Endocervical Adenocarcinoma: Clinicopathological Characteristics for Distinguishing from Primary Ovarian Mucinous or Endometrioid Tumor.In Vivo. 2024 Jul-Aug;38(4):1973-1983. doi: 10.21873/invivo.13654. In Vivo. 2024. PMID: 38936897 Free PMC article.
References
-
- Stolnicu S., Park K.J., Kiyokawa T., Oliva E., McCluggage W.G., Soslow R.A. Tumor typing of endocervical adenocarcinoma: Contemporary review and recommendations from the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2021;40:S75–S91. doi: 10.1097/PGP.0000000000000751. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

