The Combination of Radiation with PARP Inhibition Enhances Senescence and Sensitivity to the Senolytic, Navitoclax, in Triple Negative Breast Tumor Cells
- PMID: 38002066
- PMCID: PMC10669784
- DOI: 10.3390/biomedicines11113066
The Combination of Radiation with PARP Inhibition Enhances Senescence and Sensitivity to the Senolytic, Navitoclax, in Triple Negative Breast Tumor Cells
Abstract
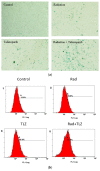
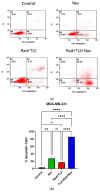
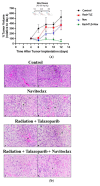
Despite significant advances in the treatment of triple-negative breast cancer, this disease continues to pose a clinical challenge, with many patients ultimately suffering from relapse. Tumor cells that recover after entering into a state of senescence after chemotherapy or radiation have been shown to develop a more aggressive phenotype, and to contribute to disease recurrence. By combining the PARP inhibitor (PARPi), talazoparib, with radiation, senescence was enhanced in 4T1 and MDA-MB-231 triple-negative breast cancer cell lines (based on SA-β-gal upregulation, increased expression of CDKN1A and the senescence-associated secretory phenotype (SASP) marker, IL6). Subsequent treatment of the radiation- and talazoparib-induced senescent 4T1 and MDA-MB231 cells with navitoclax (ABT-263) resulted in significant apoptotic cell death. In immunocompetent tumor-bearing mice, navitoclax exerted a modest growth inhibitory effect when used alone, but dramatically interfered with the recovery of 4T1-derived tumors induced into senescence with ionizing radiation and talazoparib. These findings support the potential utility of a senolytic strategy in combination with the radiotherapy/PARPi combination to mitigate the risk of disease recurrence in triple-negative breast cancer.
Keywords: PARP inhibitors; apoptosis; breast cancer; radiotherapy; senescence; senolytics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Dyba T., Randi G., Bray F., Martos C., Giusti F., Nicholson N., Gavin A., Flego M., Neamtiu L., Dimitrova N., et al. The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers. Eur. J. Cancer. 2021;157:308–347. doi: 10.1016/j.ejca.2021.07.039. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

