Synthesis, Cytotoxic, and Computational Screening of Some Novel Indole-1,2,4-Triazole-Based S-Alkylated N-Aryl Acetamides
- PMID: 38002078
- PMCID: PMC10669176
- DOI: 10.3390/biomedicines11113078
Synthesis, Cytotoxic, and Computational Screening of Some Novel Indole-1,2,4-Triazole-Based S-Alkylated N-Aryl Acetamides
Abstract
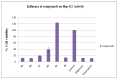
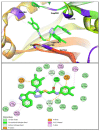
Molecular hybridization has emerged as the prime and most significant approach for the development of novel anticancer chemotherapeutic agents for combating cancer. In this pursuit, a novel series of indole-1,2,4-triazol-based N-phenyl acetamide structural motifs 8a-f were synthesized and screened against the in vitro hepatocellular cancer Hep-G2 cell line. The MTT assay was applied to determine the anti-proliferative potential of novel indole-triazole compounds 8a-f, which displayed cytotoxicity potential as cell viabilities at 100 µg/mL concentration, by using ellipticine and doxorubicin as standard reference drugs. The remarkable prominent bioactive structural hybrids 8a, 8c, and 8f demonstrated good-to-excellent anti-Hep-G2 cancer chemotherapeutic potential, with a cell viability of (11.72 ± 0.53), (18.92 ± 1.48), and (12.93 ± 0.55), respectively. The excellent cytotoxicity efficacy against the liver cancer cell line Hep-G2 was displayed by the 3,4-dichloro moiety containing indole-triazole scaffold 8b, which had the lowest cell viability (10.99 ± 0.59) compared with the standard drug ellipticine (cell viability = 11.5 ± 0.55) but displayed comparable potency in comparison with the standard drug doxorubicin (cell viability = 10.8 ± 0.41). The structure-activity relationship (SAR) of indole-triazoles 8a-f revealed that the 3,4-dichlorophenyl-based indole-triazole structural hybrid 8b displayed excellent anti-Hep-G2 cancer chemotherapeutic efficacy. The in silico approaches such as molecular docking scores, molecular dynamic simulation stability data, DFT, ADMET studies, and in vitro pharmacological profile clearly indicated that indole-triazole scaffold 8b could be the lead anti-Hep-G2 liver cancer therapeutic agent and a promising anti-Hep-G2 drug candidate for further clinical evaluations.
Keywords: ADMET studies; DFT studies; Hep-G2 cancer cell line; SAR; anticancer; hemolysis; in silico profiling; indole–triazole; thrombolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



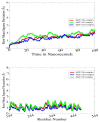

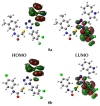

References
-
- El-Shershaby M.H., Ghiaty A., Bayoumi A.H., Ahmed H.E., El-Zoghbi M.S., El-Adl K., Abulkhair H.S. 1, 2, 4-Triazolo [4,3-c] quinazolines: A bioisosterism-guided approach towards the development of novel PCAF inhibitors with potential anticancer activity. New J. Chem. 2021;45:11136–11152. doi: 10.1039/D1NJ00710F. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

