The Role of Soluble Adenylyl Cyclase in the Regulation of Flagellar Motility in Ascidian Sperm
- PMID: 38002275
- PMCID: PMC10668965
- DOI: 10.3390/biom13111594
The Role of Soluble Adenylyl Cyclase in the Regulation of Flagellar Motility in Ascidian Sperm
Abstract
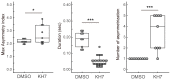
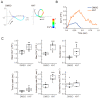
Flagellar motility in sperm is activated and regulated by factors related to the eggs at fertilization. In the ascidian Ciona intestinalis, a sulfated steroid called the SAAF (sperm activating and attracting factor) induces both sperm motility activation and chemotaxis. Cyclic AMP (cAMP) is one of the most important intracellular factors in the sperm signaling pathway. Adenylyl cyclase (AC) is the key enzyme that synthesizes cAMP at the onset of the signaling pathway in all cellular functions. We previously reported that both transmembrane AC (tmAC) and soluble AC (sAC) play important roles in sperm motility in Ciona. The tmAC plays a major role in the SAAF-induced activation of sperm motility. On the other hand, sAC is involved in the regulation of flagellar beat frequency and the Ca2+-dependent chemotactic movement of sperm. In this study, we focused on the role of sAC in the regulation of flagellar motility in Ciona sperm chemotaxis. The immunochemical analysis revealed that several isoforms of sAC protein were expressed in Ciona sperm, as reported in mammals and sea urchins. We demonstrated that sAC inhibition caused strong and transient asymmetrization during the chemotactic turn, and then sperm failed to turn toward the SAAF. In addition, real-time Ca2+ imaging in sperm flagella revealed that sAC inhibition induced an excessive and prolonged Ca2+ influx to flagella. These results indicate that sAC plays a key role in sperm chemotaxis by regulating the clearance of [Ca2+]i and by modulating Ca2+-dependent flagellar waveform conversion.
Keywords: calcium; cilia; protein kinase; sperm motility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Distinct roles of soluble and transmembrane adenylyl cyclases in the regulation of flagellar motility in Ciona sperm.Int J Mol Sci. 2014 Jul 28;15(8):13192-208. doi: 10.3390/ijms150813192. Int J Mol Sci. 2014. PMID: 25073090 Free PMC article.
-
The Roles of Two CNG Channels in the Regulation of Ascidian Sperm Chemotaxis.Int J Mol Sci. 2022 Jan 31;23(3):1648. doi: 10.3390/ijms23031648. Int J Mol Sci. 2022. PMID: 35163568 Free PMC article.
-
Lipid rafts function in Ca2+ signaling responsible for activation of sperm motility and chemotaxis in the ascidian Ciona intestinalis.Mol Reprod Dev. 2011 Dec;78(12):920-9. doi: 10.1002/mrd.21382. Epub 2011 Sep 1. Mol Reprod Dev. 2011. PMID: 21887722
-
Soluble adenylyl cyclase of sea urchin spermatozoa.Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2621-8. doi: 10.1016/j.bbadis.2014.07.011. Epub 2014 Jul 23. Biochim Biophys Acta. 2014. PMID: 25064590 Free PMC article. Review.
-
Cyclic adenosine 3',5' monophosphate, calcium and protein phosphorylation in flagellar motility.Biol Reprod. 1983 Feb;28(1):75-104. doi: 10.1095/biolreprod28.1.75. Biol Reprod. 1983. PMID: 6299416 Review.
Cited by
-
Harnessing the power of miRNAs for precision diagnosis and treatment of male infertility.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):3271-3296. doi: 10.1007/s00210-024-03594-7. Epub 2024 Nov 13. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39535597 Review.
-
Activation of motility and chemotaxis in the spermatozoa.Reprod Med Biol. 2025 Mar 5;24(1):e12638. doi: 10.1002/rmb2.12638. eCollection 2025 Jan-Dec. Reprod Med Biol. 2025. PMID: 40045950 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

