Pyrazolo-triazolo-pyrimidine Scaffold as a Molecular Passepartout for the Pan-Recognition of Human Adenosine Receptors
- PMID: 38002292
- PMCID: PMC10669182
- DOI: 10.3390/biom13111610
Pyrazolo-triazolo-pyrimidine Scaffold as a Molecular Passepartout for the Pan-Recognition of Human Adenosine Receptors
Abstract
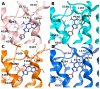
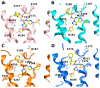
Adenosine receptors are largely distributed in our organism and are promising therapeutic targets for the treatment of many pathologies. In this perspective, investigating the structural features of the ligands leading to affinity and/or selectivity is of great interest. In this work, we have focused on a small series of pyrazolo-triazolo-pyrimidine antagonists substituted in positions 2, 5, and N8, where bulky acyl moieties at the N5 position and small alkyl groups at the N8 position are associated with affinity and selectivity at the A3 adenosine receptor even if a good affinity toward the A2B adenosine receptor has also been observed. Conversely, a free amino function at the 5 position induces high affinity at the A2A and A1 receptors with selectivity vs. the A3 subtype. A molecular modeling study suggests that differences in affinity toward A1, A2A, and A3 receptors could be ascribed to two residues: one in the EL2, E168 in human A2A/E172 in human A1, that is occupied by the hydrophobic residue V169 in the human A3 receptor; and the other in TM6, occupied by H250/H251 in human A2A and A1 receptors and by a less bulky S247 in the A3 receptor. In the end, these findings could help to design new subtype-selective adenosine receptor ligands.
Keywords: GPCR; adenosine receptor; antagonists; molecular modeling; pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Design, synthesis, and biological evaluation of C9- and C2-substituted pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines as new A2A and A3 adenosine receptors antagonists.J Med Chem. 2003 Mar 27;46(7):1229-41. doi: 10.1021/jm021023m. J Med Chem. 2003. PMID: 12646033
-
Pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives as adenosine receptor antagonists. Influence of the N5 substituent on the affinity at the human A 3 and A 2B adenosine receptor subtypes: a molecular modeling investigation.J Med Chem. 2003 Sep 25;46(20):4287-96. doi: 10.1021/jm030852k. J Med Chem. 2003. PMID: 13678407
-
Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes.J Med Chem. 1998 Jul 16;41(15):2835-45. doi: 10.1021/jm980094b. J Med Chem. 1998. PMID: 9667972 Free PMC article.
-
Pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidines and Structurally Simplified Analogs. Chemistry and SAR Profile as Adenosine Receptor Antagonists.Curr Top Med Chem. 2016;16(28):3224-3257. doi: 10.2174/1568026616666160506145831. Curr Top Med Chem. 2016. PMID: 27150365 Review.
-
Selective adenosine A2A receptor antagonists.Farmaco. 2001 Jan-Feb;56(1-2):87-90. doi: 10.1016/s0014-827x(01)01024-2. Farmaco. 2001. PMID: 11347973 Review.
References
-
- Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Bolis L., Straub R.W., editors. Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach. Raven Press; New York, NY, USA: 1978. pp. 107–118.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

