Structural and Functional Insights into the Stealth Protein CpsY of Mycobacterium tuberculosis
- PMID: 38002293
- PMCID: PMC10668966
- DOI: 10.3390/biom13111611
Structural and Functional Insights into the Stealth Protein CpsY of Mycobacterium tuberculosis
Abstract
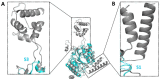
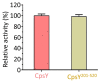
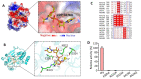
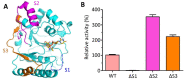
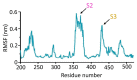
Mycobacterium tuberculosis (Mtb) is an important and harmful intracellular pathogen that is responsible for the cause of tuberculosis (TB). Mtb capsular polysaccharides can misdirect the host's immune response pathways, resulting in additional challenges in TB treatment. These capsule polysaccharides are biosynthesized by stealth proteins, including CpsY. The structure and functional mechanism of Mtb CpsY are not completely delineated. Here, we reported the crystal structure of CpsY201-520 at 1.64 Å. CpsY201-520 comprises three β-sheets with five α-helices on one side and three on the other. Four conserved regions (CR1-CR4) are located near and at the base of its catalytic cavity, and three spacer segments (S1-S3) surround the catalytic cavity. Site-directed mutagenesis demonstrated the strict conservation of R419 at CR3 and S1-S3 in regulating the phosphotransferase activity of CpsY201-520. In addition, deletion of S2 or S3 (∆S2 or ∆S3) dramatically increased the activity compared to the wild-type (WT) CpsY201-520. Results from molecular dynamics (MD) simulations showed that S2 and S3 are highly flexible. Our study provides new insights for the development of new vaccines and targeted immunotherapy against Mtb.
Keywords: Mycobacterium tuberculosis; crystal structure; phosphotransferase activity; stealth protein CpsY.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures


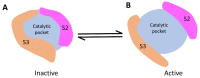
Similar articles
-
Functional and mechanistic insights into the stealth protein full-length CpsY is conducive to understanding immune evasion mechanisms by Mycobacterium tuberculosis.Tuberculosis (Edinb). 2025 May;152:102616. doi: 10.1016/j.tube.2025.102616. Epub 2025 Feb 19. Tuberculosis (Edinb). 2025. PMID: 39985825
-
Insights into RpoB clinical mutants in mediating rifampicin resistance in Mycobacterium tuberculosis.J Mol Graph Model. 2016 Jun;67:20-32. doi: 10.1016/j.jmgm.2016.04.005. Epub 2016 Apr 23. J Mol Graph Model. 2016. PMID: 27155814
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Molecular insights into the differential efflux mechanism of Rv1634 protein, a multidrug transporter of major facilitator superfamily in Mycobacterium tuberculosis.Proteins. 2022 Feb;90(2):566-578. doi: 10.1002/prot.26253. Epub 2021 Oct 19. Proteins. 2022. PMID: 34601761
-
Immunometabolism during Mycobacterium tuberculosis Infection.Trends Microbiol. 2020 Oct;28(10):832-850. doi: 10.1016/j.tim.2020.04.010. Epub 2020 May 11. Trends Microbiol. 2020. PMID: 32409147 Free PMC article. Review.
Cited by
-
Predicting the Structure of Enzymes with Metal Cofactors: The Example of [FeFe] Hydrogenases.Int J Mol Sci. 2024 Mar 25;25(7):3663. doi: 10.3390/ijms25073663. Int J Mol Sci. 2024. PMID: 38612474 Free PMC article.
References
-
- Dominguez J., Boeree M.J., Cambau E., Chesov D., Conradie F., Cox V., Dheda K., Dudnyk A., Farhat M.R., Gagneux S., et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: A 2023 TBnet/RESIST-TB consensus statement. Lancet Infect Dis. 2023;23:e122–e137. doi: 10.1016/S1473-3099(22)00875-1. - DOI - PMC - PubMed
-
- Rustage K., Lobe J., Hayward S.E., Kristensen K.L., Margineanu I., Stienstra Y., Goletti D., Zenner D., Noori T., Pareek M., et al. Initiation and completion of treatment for latent tuberculosis infection in migrants globally: A systematic review and meta-analysis. Lancet Infect. Dis. 2021;21:1701–1712. doi: 10.1016/S1473-3099(21)00052-9. - DOI - PMC - PubMed
-
- Dhana A., Hamada Y., Kengne A.P., Kerkhoff A.D., Rangaka M.X., Kredo T., Baddeley A., Miller C., Singh S., Hanifa Y., et al. Tuberculosis screening among ambulatory people living with HIV: A systematic review and individual participant data meta-analysis. Lancet Infect. Dis. 2022;22:507–518. doi: 10.1016/S1473-3099(21)00387-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

