Human Skin Aging and the Anti-Aging Properties of Retinol
- PMID: 38002296
- PMCID: PMC10669284
- DOI: 10.3390/biom13111614
Human Skin Aging and the Anti-Aging Properties of Retinol
Abstract
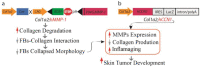
The skin is the most-extensive and -abundant tissue in the human body. Like many organs, as we age, human skin experiences gradual atrophy in both the epidermis and dermis. This can be primarily attributed to the diminishing population of epidermal stem cells and the reduction in collagen, which is the primary structural protein in the human body. The alterations occurring in the epidermis and dermis due to the aging process result in disruptions to the structure and functionality of the skin. This creates a microenvironment conducive to age-related skin conditions such as a compromised skin barrier, slowed wound healing, and the onset of skin cancer. This review emphasizes the recent molecular discoveries related to skin aging and evaluates preventive approaches, such as the use of topical retinoids. Topical retinoids have demonstrated promise in enhancing skin texture, diminishing fine lines, and augmenting the thickness of both the epidermal and dermal layers.
Keywords: MMPs; TGF-β; collagen; retinol; skin aging.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

