Trace Amine-Associated Receptors and Monoamine-Mediated Regulation of Insulin Secretion in Pancreatic Islets
- PMID: 38002300
- PMCID: PMC10669413
- DOI: 10.3390/biom13111618
Trace Amine-Associated Receptors and Monoamine-Mediated Regulation of Insulin Secretion in Pancreatic Islets
Abstract
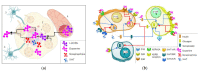
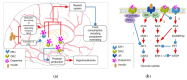
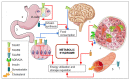
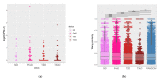
Currently, metabolic syndrome treatment includes predominantly pharmacological symptom relief and complex lifestyle changes. Trace amines and their receptor systems modulate signaling pathways of dopamine, norepinephrine, and serotonin, which are involved in the pathogenesis of this disorder. Trace amine-associated receptor 1 (TAAR1) is expressed in endocrine organs, and it was revealed that TAAR1 may regulate insulin secretion in pancreatic islet β-cells. For instance, accumulating data demonstrate the positive effect of TAAR1 agonists on the dynamics of metabolic syndrome progression and MetS-associated disease development. The role of other TAARs (TAAR2, TAAR5, TAAR6, TAAR8, and TAAR9) in the islet's function is much less studied. In this review, we summarize the evidence of TAARs' contribution to the metabolic syndrome pathogenesis and regulation of insulin secretion in pancreatic islets. Additionally, by the analysis of public transcriptomic data, we demonstrate that TAAR1 and other TAAR receptors are expressed in the pancreatic islets. We also explore associations between the expression of TAARs mRNA and other genes in studied samples and demonstrate the deregulation of TAARs' functional associations in patients with metabolic diseases compared to healthy donors.
Keywords: GPCR; TAAR; dopamine; insulin; pancreatic islets; trace amine-associated receptors; trace amines; transcriptomic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Trace Amine-Associated Receptors' Role in Immune System Functions.Biomedicines. 2024 Apr 18;12(4):893. doi: 10.3390/biomedicines12040893. Biomedicines. 2024. PMID: 38672247 Free PMC article. Review.
-
Deregulation of Trace Amine-Associated Receptors (TAAR) Expression and Signaling Mode in Melanoma.Biomolecules. 2022 Jan 11;12(1):114. doi: 10.3390/biom12010114. Biomolecules. 2022. PMID: 35053262 Free PMC article.
-
The Expression of Trace Amine-Associated Receptors (TAARs) in Breast Cancer Is Coincident with the Expression of Neuroactive Ligand-Receptor Systems and Depends on Tumor Intrinsic Subtype.Biomolecules. 2023 Sep 7;13(9):1361. doi: 10.3390/biom13091361. Biomolecules. 2023. PMID: 37759760 Free PMC article.
-
Expression of Trace Amine-Associated Receptors in the Murine and Human Hippocampus Based on Public Transcriptomic Data.Cells. 2022 Jun 1;11(11):1813. doi: 10.3390/cells11111813. Cells. 2022. PMID: 35681508 Free PMC article.
-
Trace Amines and Their Receptors.Pharmacol Rev. 2018 Jul;70(3):549-620. doi: 10.1124/pr.117.015305. Pharmacol Rev. 2018. PMID: 29941461 Review.
Cited by
-
Trace Amine-Associated Receptors' Role in Immune System Functions.Biomedicines. 2024 Apr 18;12(4):893. doi: 10.3390/biomedicines12040893. Biomedicines. 2024. PMID: 38672247 Free PMC article. Review.
-
Functional Analysis of TAAR1 Expression in the Intestine Wall and the Effect of Its Gene Knockout on the Gut Microbiota in Mice.Int J Mol Sci. 2024 Dec 9;25(23):13216. doi: 10.3390/ijms252313216. Int J Mol Sci. 2024. PMID: 39684925 Free PMC article.
-
The Neurometabolic Function of the Dopamine-Aminotransferase System.Metabolites. 2025 Jan 6;15(1):21. doi: 10.3390/metabo15010021. Metabolites. 2025. PMID: 39852364 Free PMC article. Review.
-
Knocking Out TAAR5: A Pathway to Enhanced Neurogenesis and Dopamine Signaling in the Striatum.Cells. 2024 Nov 19;13(22):1910. doi: 10.3390/cells13221910. Cells. 2024. PMID: 39594659 Free PMC article.
References
-
- Borowsky B., Adham N., Jones K.A., Raddatz R., Artymyshyn R., Ogozalek K.L., Durkin M.M., Lakhlani P.P., Bonini J.A., Pathirana S., et al. Trace Amines: Identification of a Family of Mammalian G Protein-Coupled Receptors. Proc. Natl. Acad. Sci. USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. - DOI - PMC - PubMed
-
- Bunzow J.R., Sonders M.S., Arttamangkul S., Harrison L.M., Zhang G., Quigley D.I., Darland T., Suchland K.L., Pasumamula S., Kennedy J.L., et al. Amphetamine, 3,4-Methylenedioxymethamphetamine, Lysergic Acid Diethylamide, and Metabolites of the Catecholamine Neurotransmitters Are Agonists of a Rat Trace Amine Receptor. Mol. Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. - DOI - PubMed
-
- Espinoza S., Sukhanov I., Efimova E.V., Kozlova A., Antonova K.A., Illiano P., Leo D., Merkulyeva N., Kalinina D., Musienko P., et al. Trace Amine-Associated Receptor 5 Provides Olfactory Input Into Limbic Brain Areas and Modulates Emotional Behaviors and Serotonin Transmission. Front. Mol. Neurosci. 2020;13:18. doi: 10.3389/fnmol.2020.00018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

