Good Cop, Bad Cop: Profiling the Immune Landscape in Multiple Myeloma
- PMID: 38002311
- PMCID: PMC10669790
- DOI: 10.3390/biom13111629
Good Cop, Bad Cop: Profiling the Immune Landscape in Multiple Myeloma
Abstract
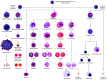
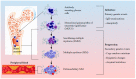
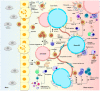
Multiple myeloma (MM) is a dyscrasia of plasma cells (PCs) characterized by abnormal immunoglobulin (Ig) production. The disease remains incurable due to a multitude of mutations and structural abnormalities in MM cells, coupled with a favorable microenvironment and immune suppression that eventually contribute to the development of drug resistance. The bone marrow microenvironment (BMME) is composed of a cellular component comprising stromal cells, endothelial cells, osteoclasts, osteoblasts, and immune cells, and a non-cellular component made of the extracellular matrix (ECM) and the liquid milieu, which contains cytokines, growth factors, and chemokines. The bone marrow stromal cells (BMSCs) are involved in the adhesion of MM cells, promote the growth, proliferation, invasion, and drug resistance of MM cells, and are also crucial in angiogenesis and the formation of lytic bone lesions. Classical immunophenotyping in combination with advanced immune profiling using single-cell sequencing technologies has enabled immune cell-specific gene expression analysis in MM to further elucidate the roles of specific immune cell fractions from peripheral blood and bone marrow (BM) in myelomagenesis and progression, immune evasion and exhaustion mechanisms, and development of drug resistance and relapse. The review describes the role of BMME components in MM development and ongoing clinical trials using immunotherapeutic approaches.
Keywords: hematopoiesis; immune profiling; immunotherapy; multiple myeloma; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The impact of the bone marrow microenvironment on multiple myeloma (Review).Oncol Rep. 2019 Oct;42(4):1272-1282. doi: 10.3892/or.2019.7261. Epub 2019 Aug 5. Oncol Rep. 2019. PMID: 31524246
-
Bone marrow microenvironment in multiple myeloma progression.J Biomed Biotechnol. 2012;2012:157496. doi: 10.1155/2012/157496. Epub 2012 Oct 3. J Biomed Biotechnol. 2012. PMID: 23093834 Free PMC article. Review.
-
The Role of Marrow Microenvironment in the Growth and Development of Malignant Plasma Cells in Multiple Myeloma.Int J Mol Sci. 2021 Apr 24;22(9):4462. doi: 10.3390/ijms22094462. Int J Mol Sci. 2021. PMID: 33923357 Free PMC article. Review.
-
Predicting the impact of combined therapies on myeloma cell growth using a hybrid multi-scale agent-based model.Oncotarget. 2017 Jan 31;8(5):7647-7665. doi: 10.18632/oncotarget.13831. Oncotarget. 2017. PMID: 28032590 Free PMC article.
-
Osteoclast Immunosuppressive Effects in Multiple Myeloma: Role of Programmed Cell Death Ligand 1.Front Immunol. 2018 Aug 10;9:1822. doi: 10.3389/fimmu.2018.01822. eCollection 2018. Front Immunol. 2018. PMID: 30147691 Free PMC article. Review.
Cited by
-
Peripheral inflammatory T cell subsets are effective predictive factors in the development of heterotopic ossification after posttraumatic elbow surgery.Heliyon. 2024 Jul 2;10(13):e33851. doi: 10.1016/j.heliyon.2024.e33851. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39055826 Free PMC article.
-
Pathways to therapy resistance: The sheltering effect of the bone marrow microenvironment to multiple myeloma cells.Heliyon. 2024 Jun 14;10(12):e33091. doi: 10.1016/j.heliyon.2024.e33091. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39021902 Free PMC article. Review.
-
Using protein turnover assay to explore the drug mechanism of Carfilzomib.Acta Biochim Biophys Sin (Shanghai). 2024 Jul 8;57(2):209-222. doi: 10.3724/abbs.2024104. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38978505 Free PMC article.
-
Topography-based implants for bone regeneration: Design, biological mechanism, and therapeutics.Mater Today Bio. 2025 Jul 13;34:102066. doi: 10.1016/j.mtbio.2025.102066. eCollection 2025 Oct. Mater Today Bio. 2025. PMID: 40735704 Free PMC article. Review.
-
The prognostic value of the platelet-to-lymphocyte ratio in multiple myeloma patients treated with a bortezomib-based regimen.Sci Rep. 2025 Jan 13;15(1):1819. doi: 10.1038/s41598-024-84343-x. Sci Rep. 2025. PMID: 39805912 Free PMC article.
References
-
- Godin I., Cumano A. Hematopoietic Stem Cell Development. Springer Science & Business Media; Berlin, Germany: 2010. 178p
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

